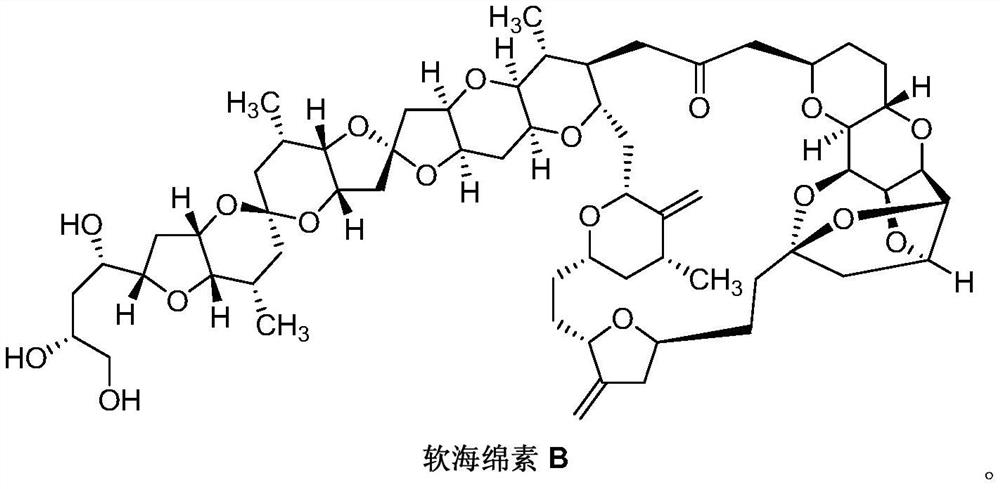
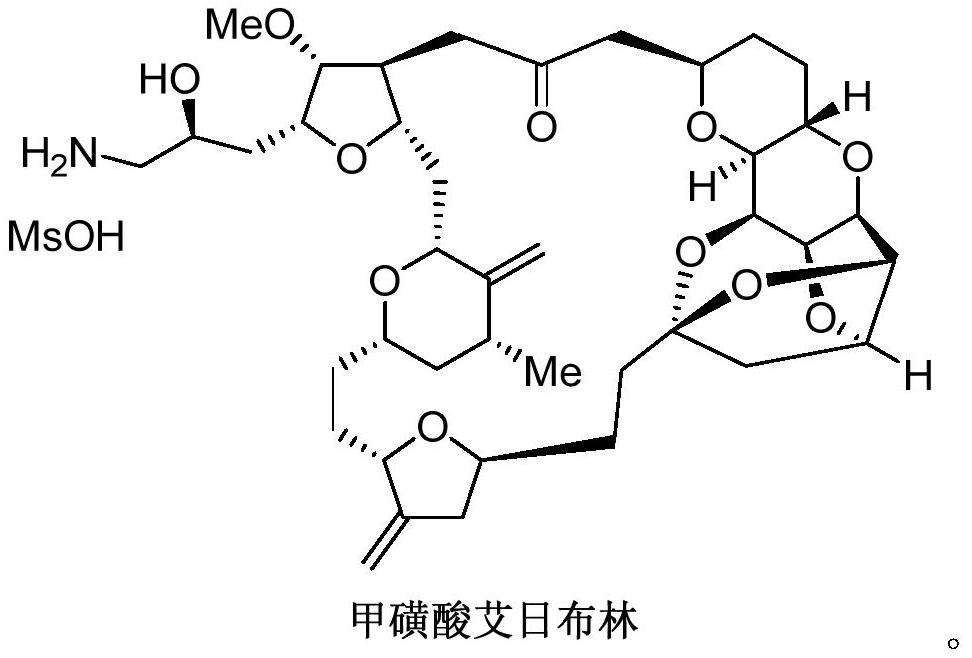
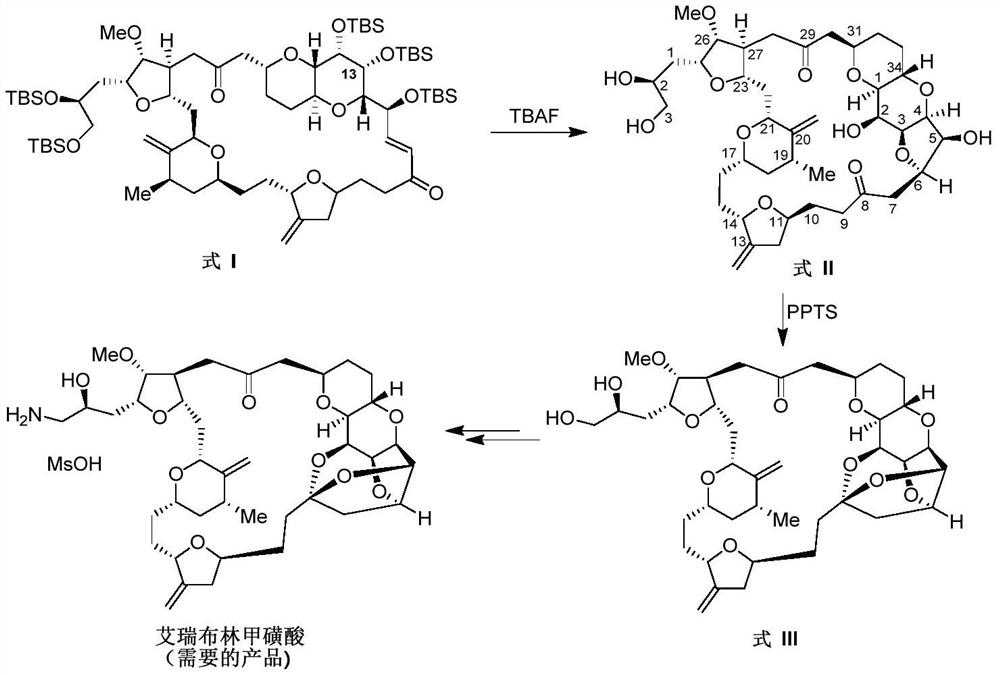
Synthesis of Eribulin Mesylate
A technology of methanesulfonic acid and compounds, which is applied in the field of preparation of key intermediates of the complex natural modified drug Eribulin mesilate, can solve the problems of slow research and development progress and limited sample volume, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment one: the preparation of formula II compound (using piperidine hydrochloride) (yield 85%)
[0017]
[0018] Dissolve the compound of formula I (460.0mg, 0.348mmol) in THF (21.5mL) at 20-25°C, add piperidine hydrochloride (211.6mg, 1.74mmol) and TBAF (1M THF solution, 3.48mL, 3.48mmol ), keep stirring at this temperature for 20h. The reaction solution was concentrated under reduced pressure at 30°C to remove THF, then dissolved in a small amount of DCM, and purified by column chromatography (EA / n-Hept=1 / 1→EA→MeOH / EA=1 / 15) to obtain the target compound of formula II (white solid, 222.3 mg, 85.3%). ESI-MS:C 40 h 60 o 13 Calculated value: 748.4034, measured value: 749.4065 (M+H + ). 1 H NMR (600MHz, CDCl 3 )5.00 (1H, brd, J = 1.8Hz), 4.85 (1H, brd, J = 1.8Hz), 4.88 (1H, brs), 4.80 (1H, brd, J = 1.2Hz), 4.48-4.54 (1H, m),4.38(1H,dd,J 1 =9.0Hz,J 2 =4.2Hz), 4.34(1H,dt,J 1 =10.2Hz,J 2 =3.6Hz),3.97-4.09(5H,m),3.94-3.99(1H,m),3.85-3.94(3H,m),3.78-3.85(1H,...
Embodiment 2
[0019] Embodiment two: the preparation of formula II compound (using pyridine hydrochloride) (yield 89%)
[0020]
[0021] Dissolve the compound of formula I (460.0mg, 0.348mmol) in THF (21.5mL) at 20-25°C, add pyridine hydrochloride (201.1mg, 1.74mmol) and TBAF (1M THF solution, 3.48mL, 3.48mmol) , Keep stirring at this temperature for 20h. The reaction solution was concentrated under reduced pressure at 30°C to remove THF, then dissolved in a small amount of DCM, and purified by column chromatography (EA / n-Hept=1 / 1→EA→MeOH / EA=1 / 15) to obtain the target compound of formula II (white solid, 232.1 mg, 88.9%).
Embodiment 3
[0022] Embodiment three: the preparation of formula II compound (comparative experiment, only uses TBAF) (yield 38%)
[0023]
[0024]Dissolve the compound of formula I (460.0mg, 0.348mmol) in THF (21.5mL) at 20-25°C, add TBAF (1M THF solution, 3.48mL, 3.48mmol), and keep stirring at this temperature for 20h. The reaction solution was concentrated under reduced pressure at 30°C to remove THF, then dissolved in a small amount of DCM, and purified by column chromatography (EA / n-Hept=1 / 1→EA→MeOH / EA=1 / 15) to obtain the target compound of formula II (white solid, 99.7mg, 38.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com