A kind of method for the determination of sodium lauryl sarcosinate content by high performance liquid chromatography
A technology of sodium lauryl sarcosine and high performance liquid chromatography, which is applied in the field of detection, can solve problems such as inaccurate measurement results, and achieve the effects of wide application range, rapid separation, and accurate and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of Solutions
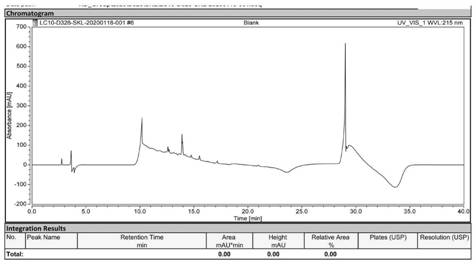
[0048] Precisely weigh 1.00 g of sodium lauryl sarcosinate reference substance, dissolve it in ultrapure water, and use a volumetric flask to dilute to 100 mL to obtain a 10 mg / mL sodium lauryl sarcosinate reference substance solution; Amount of bovine serum albumin sample containing lauryl sarcosine ammonia 5.00g, according to the estimated content of sodium lauryl sarcosinate, diluted with ultrapure water to the content of sodium lauryl sarcosinate in Between 0.05-2.00 mg / mL, a sample solution to be tested containing sodium lauryl sarcosinate was obtained; water for injection (WFI) was used as a blank control.
[0049] 2. Chromatographic conditions
[0050] Measure an appropriate amount of ultrapure water, add 1 mL of trifluoroacetic acid, add ultrapure water to 1000 mL, and filter with a 0.22 μm membrane to obtain a 0.1% trifluoroacetic acid-water solution as mobile phase A; weigh an appropriate amount of acetonitrile, add 0.85 mL The ...
Embodiment 2
[0057] The preparation of the solution, mobile phase A, mobile phase B, gradient elution procedure and chromatographic column are the same as in Example 1. The injection volume was 52 μL, the flow rate was 1.2 mL / min, and the detection wavelength was 218 nm. There are chromatographic peaks with the same retention time at the corresponding positions of the chromatograms of the reference solution and the sample solution to be tested.
Embodiment 3
[0059] 3.1 Solution preparation and chromatographic conditions
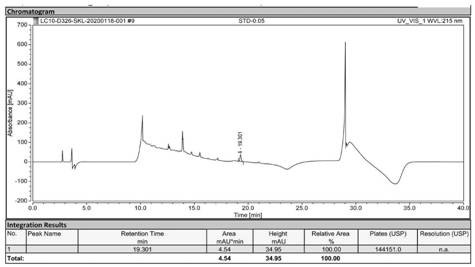
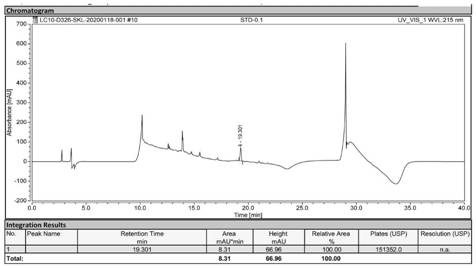
[0060] Precisely weigh 1.00 g of sodium lauryl sarcosinate, dissolve it in ultrapure water, and dilute the volume to 100 mL in a volumetric flask to obtain a reference solution of 10.00 mg / mL. When injecting, the mass concentration is 10.00 mg / mL 5 μL, 10 μL, 25 μL, 50 μL, 100 μL, 150 μL and 200 μL of the reference solution were injected respectively, that is, 0.05 mg / mL, 0.10 mg / mL, 0.25 mg / mL, 0.50 mg / mL, 1.00 mg / mL and 1.50 mg / mL were obtained respectively. mg / mL and 2.00 mg / mL reference solutions, labeled STD-0.05, STD-0.10, STD-0.25, STD-0.50, STD-1.00, STD-1.50, and STD-2.00.
[0061] According to the estimated content of sodium lauryl sarcosinate in a batch of ApoJ samples (referred to as sample 1 to be tested) and another batch of ApoJ samples (referred to as sample 2 to be tested), use ultrapure water Dilute until the sodium lauryl sarcosinate content is between 0.05-2.00 mg / mL.
[0062] The quality co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com