Application of metformin in preparation of medicine for treating eye melanoma
A technology for metformin and melanoma, which is used in antitumor drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
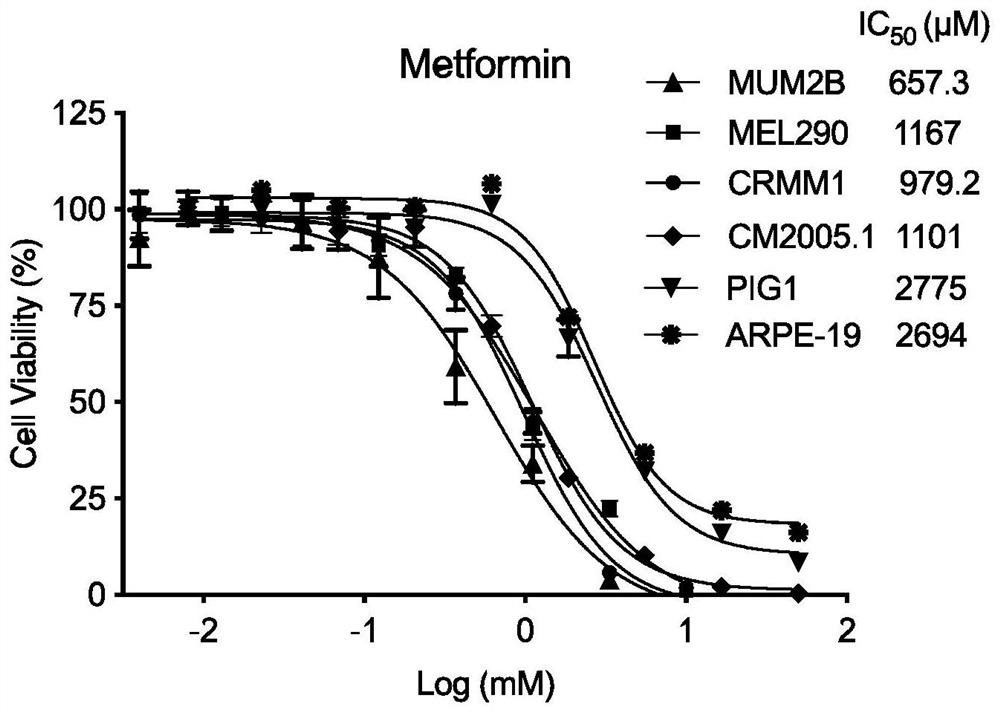
[0020] Embodiment 1 IC50 determination experiment
[0021] Experimental materials: human uveal melanoma cell lines MUM2B, MEL290, human conjunctival melanoma cell lines CRMM1, CM2005.1, human normal melanocyte PIG1, human retinal pigment epithelial cell ARPE-19. Metformin (Metformin) was purchased from Selleck (China), and CCK8 was purchased from Tongren Chemical (Japan).
[0022] Experimental steps: (1) MUM2B, MEL290, CRMM1, CM2005.1, PIG1, ARPE-19 cells were routinely cultured in a 37°C, 5% CO2 incubator. MUM2B, MEL290, and ARPE-19 were cultured in DMEM with 10% FBS, and CRMM1, CM2005.1, and PIG1 were cultured in F12-K with 10% FBS.
[0023] (2) The cells were cultured to a density of 70%-80%. When the refractive index was high, they were digested with trypsin, centrifuged at 800rpm for 4min, the supernatant was removed, and resuspended with 2ml of complete medium. Pipette evenly, take 20 μl and count with a cell counting plate, inoculate 3000 cells per well in a 384-well ...
Embodiment 2
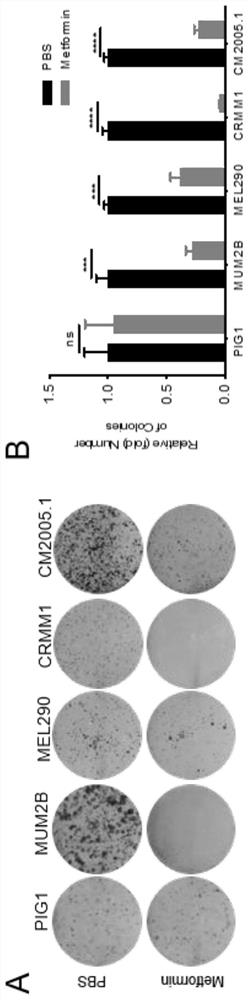
[0025] Example 2 Plate colony formation experiment
[0026] Experimental materials: human uveal melanoma cell lines MUM2B, MEL290, human conjunctival melanoma cell lines CRMM1, CM2005.1, human normal melanocyte PIG1. Metformin (Metformin) was purchased from Selleck (China), pancreatin was purchased from Xinsaimei Biotech (China), 4% paraformaldehyde and crystal violet dye were purchased from Shenggong Company (China).
[0027] Experimental steps: (1) MUM2B, MEL290, CRMM1, CM2005.1, and PIG1 cells were conventionally cultured in a 5% CO2 incubator at 37°C. MUM2B and MEL290 were cultured in DMEM with 10% FBS, and CRMM1, CM2005.1 and PIG1 were cultured in F12-K with 10% FBS.
[0028] (2) The cells were cultured to a density of 70%-80%. When the refractive index was high, they were digested with trypsin, centrifuged at 800rpm for 4min, the supernatant was removed, and resuspended with 2ml of complete medium. Pipette evenly, take 20 μl and count with a cell counting plate, take 1...
Embodiment 3
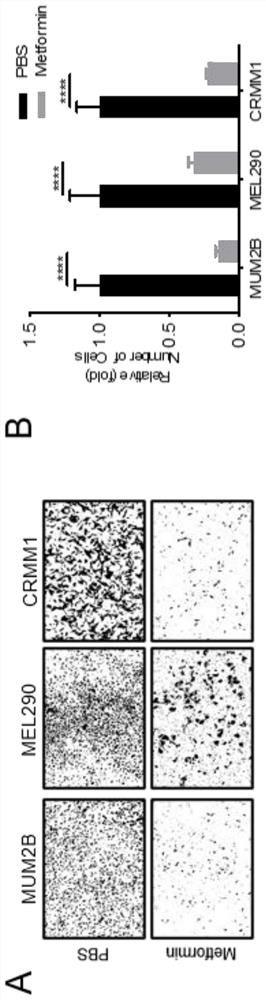
[0030] Example 3 Transwell cell migration experiment
[0031]Experimental materials: human uveal melanoma cell lines MUM2B, MEL290, human conjunctival melanoma cell line CRMM1. Metformin was purchased from Selleck (China), 24-well plates were purchased from Thermo Fisher (USA), and 24-well plate Transwell chambers were purchased from Millipore (USA).
[0032] Experimental steps: (1) MUM2B, MEL290, CRMM1, CM2005.1, and PIG1 cells were conventionally cultured in a 5% CO2 incubator at 37°C. MUM2B and MEL290 were cultured in DMEM with 10% FBS, and CRMM1, CM2005.1 and PIG1 were cultured in F12-K with 10% FBS.
[0033] (2) The cells were cultured to a density of 70%-80%. When the refractive index was high, they were digested with trypsin, centrifuged at 800rpm for 4min, the supernatant was removed, and resuspended with 2ml of complete medium. Pipette evenly, take 20 μl and count with a cell counting plate. Add 900 μl of 10% FBS medium to each well of a 24-well plate, hang 8 μm Tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com