Modified membrane for blood purification and preparation method thereof
A blood purification and modification technology, applied in chemical instruments and methods, blood filtration, membrane technology, etc., can solve the problem of less attention to the anti-inflammatory effect of membrane materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] Specifically, the preparation method of a blood purification modified membrane provided by this application includes the following steps:
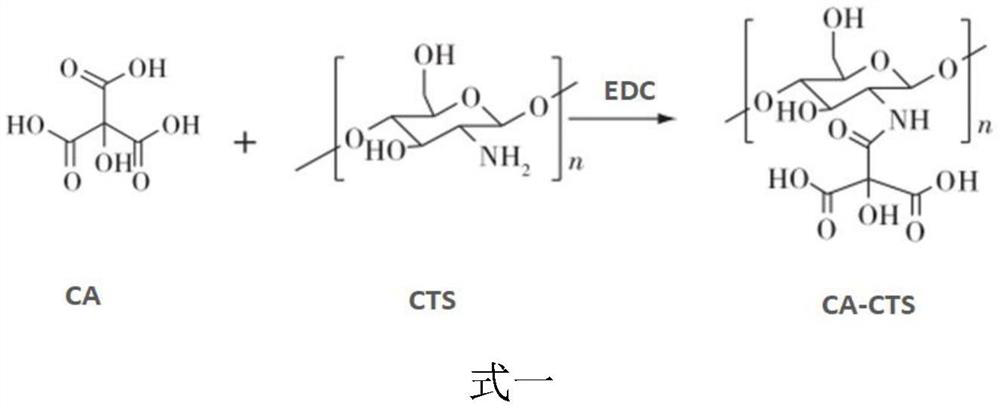
[0045] (1) Preparation of citric acid-coupled chitosan (CA-CTS): citric acid (CA) and EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide) Dissolve in anhydrous DMSO (dimethyl sulfoxide), stir until dissolved, add CTS (chitosan)-acetic acid-sodium acetate buffer, stir for 12-48 hours to obtain a mixed solution; adjust the concentration of the mixed solution with NaOH pH to 8.0-9.0, filter, and take the precipitate to obtain citric acid-coupled chitosan (CA-CTS); the mass ratio of the citric acid to the EDC is (100-150): (15-20) , each milliliter of the DMSO contains 6.67-10 mg of citric acid;
[0046] (2) Preparation of CA-CTS-loaded ticagrelor microspheres: Dissolve the citric acid-coupled chitosan (CA-CTS) in step (1) in acetic acid solution, then add ticagrelor, and stir well , to obtain a mixed solution; dropwise add liquid par...
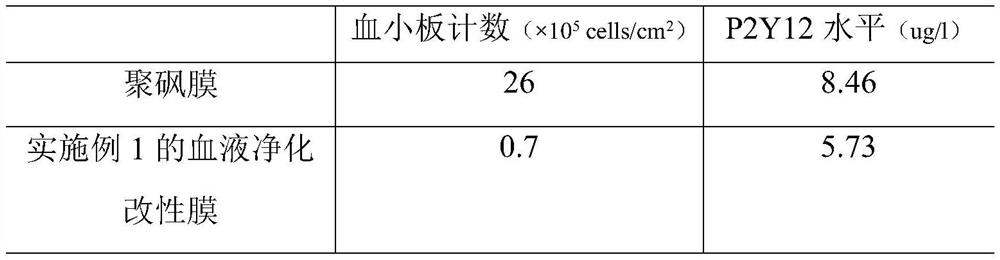
Embodiment 1
[0069] (1) Preparation of citric acid-coupled chitosan (CA-CTS)
[0070] Dissolve 150 mg of citric acid and 20 mg of EDC in 15 ml of anhydrous DMSO, stir magnetically at room temperature and protect from light until completely dissolved, then add 50 ml of 5 mg / ml CTS acetic acid-sodium acetate buffer (pH5.0), stir magnetically at room temperature and protect from light for 24 hours, The mixed solution was obtained; the pH of the mixed solution was adjusted to 9.0 with NaOH, filtered, the precipitate was taken, washed 3 times with distilled water, and vacuum-dried for 24 hours to obtain citric acid-coupled chitosan (CA-CTS);
[0071] (2) Preparation of CA-CTS loaded ticagrelor microspheres (prepared by emulsification cross-linking method)
[0072] Dissolve 90 mg of CA-CTS in step (1) in 5 ml of 2% acetic acid solution, then add 30 mg of ticagrelor and stir evenly to obtain a mixed solution; slowly drop into the mixed solution containing 6 g of Span 80 120ml of liquid paraffin,...
Embodiment 2
[0076] (1) Preparation of citric acid-coupled chitosan (CA-CTS)
[0077] Dissolve 100 mg of citric acid and 15 mg of EDC in 15 ml of anhydrous DMSO, stir magnetically at room temperature and protect from light until completely dissolved, then add 30 ml of 5 mg / ml CTS acetic acid-sodium acetate buffer (pH4.0), stir magnetically at room temperature and protect from light for 12 hours, The mixed solution was obtained; the pH of the mixed solution was adjusted to 8.0 with NaOH, filtered, the precipitate was taken, washed 3 times with distilled water, and dried in vacuum for 24 hours to obtain citric acid-coupled chitosan (CA-CTS);
[0078] (2) Preparation of CA-CTS loaded ticagrelor microspheres (prepared by emulsification cross-linking method)
[0079] Dissolve 60 mg of CA-CTS in step (1) in 5 ml of 2% acetic acid solution, then add 20 mg of ticagrelor and stir evenly to obtain a mixed solution; slowly drop into the mixed solution containing 3 g of Span 80 120ml of liquid paraff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com