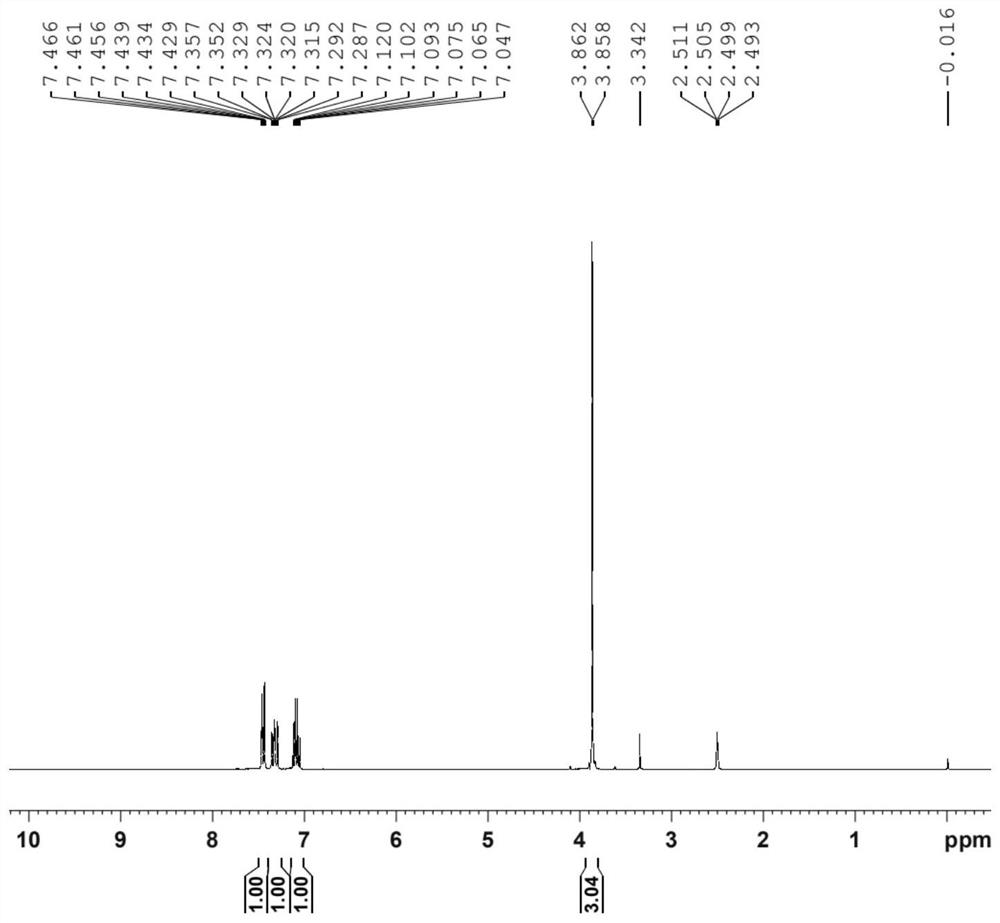
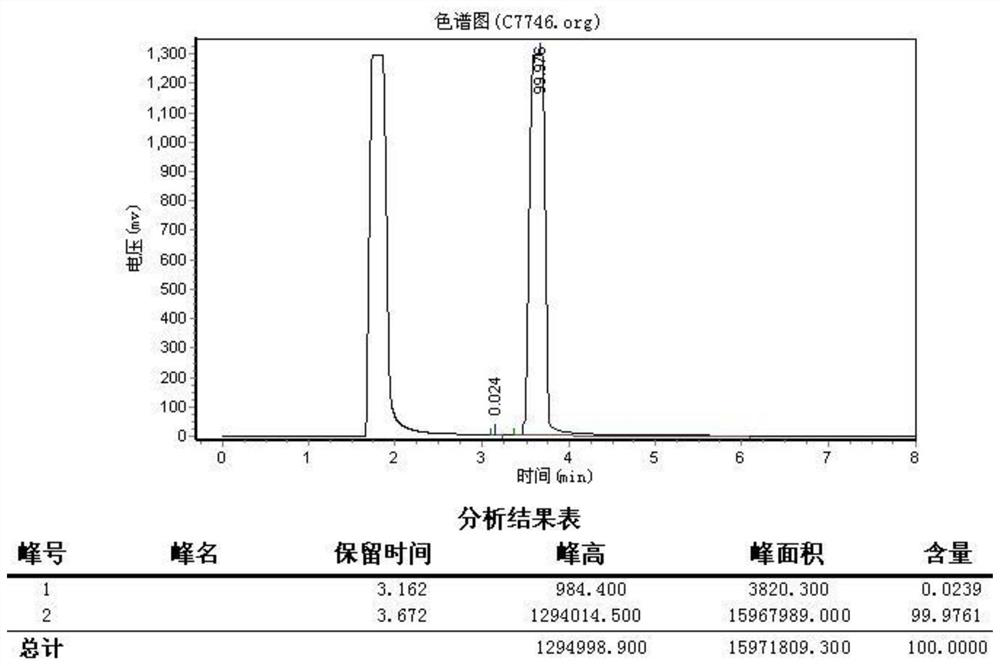
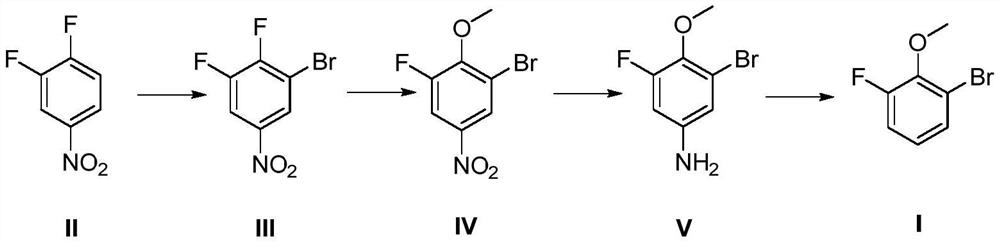
Preparation method of 2-bromo-6-fluoroanisole
A technology of fluoroanisole and difluoronitrobenzene, which is applied in the field of preparation of 2-bromo-6-fluoroanisole, can solve the problems that are not conducive to industrial scale-up and generation, and a large amount of waste water, and achieve low price and easy disposal , the effect of little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of 2-bromo-6-fluoroanisole, the preparation method comprises the steps:
[0027] (1) Synthesis of compound III
[0028] Add 159g (1mol) 3,4-difluoronitrobenzene, 500g concentrated sulfuric acid (mass concentration 98%) to the reaction flask, add 195.8g (1.1mol) bromosuccinimide in batches at 40°C, and finish adding Keep stirring for 12 hours, follow the reaction by HPLC until the reaction of 3,4-difluoronitrobenzene is complete; quench into 2000ml ice water, filter, and recrystallize the filter cake with acetone-water system to obtain 194.3g of compound III, molar yield: 82 %.
[0029] (2) Synthesis of Compound IV
[0030] Add 118.5g (0.5mol) compound III, 700ml methanol, 29.7g (0.55mol) sodium methoxide into the reaction flask, stir at 60°C for 6 hours, follow the reaction by HPLC until the compound III reaction is complete; quench into 2000ml ice water, Filter and dry to obtain 103.3 g of compound IV, molar yield: 83%.
[0031] (3) Synthesis o...
Embodiment 2
[0038] A preparation method of 2-bromo-6-fluoroanisole, the preparation method comprises the steps:
[0039] (1) Synthesis of compound III
[0040] To the reaction flask, add 159g (1mol) 3,4-difluoronitrobenzene, 500g concentrated sulfuric acid (mass concentration 98%), add 231.4g (1.3mol) bromosuccinimide in batches at 60°C, and finish adding Keep stirring for 6 hours, follow the reaction by HPLC until the reaction of 3,4-difluoronitrobenzene is complete; quench into 2000ml ice water, filter, and recrystallize the filter cake with acetone-water system to obtain 199.1g of compound III, molar yield: 84 %.
[0041] (2) Synthesis of Compound IV
[0042] Add 118.5g (0.5mol) of compound III, 700ml of methanol, 35.1g (0.65mol) of sodium methoxide into the reaction flask, stir at 50°C for 4 hours, follow the reaction by HPLC until the reaction of compound III is complete; quench into 2000ml of ice water, Filter and dry to obtain 109.5 g of compound IV, molar yield: 88%.
[0043] ...
Embodiment 3
[0048] A preparation method of 2-bromo-6-fluoroanisole, the preparation method comprises the steps:
[0049] (1) Synthesis of compound III
[0050] To the reaction flask, add 159g (1mol) 3,4-difluoronitrobenzene, 500g concentrated sulfuric acid (mass concentration 98%), add 267g (1.5mol) bromosuccinimide in batches at 20°C, add the insulation Stir for 12 hours, follow the reaction by HPLC until the reaction of 3,4-difluoronitrobenzene is complete; quench into 2000ml of ice water, filter, and recrystallize the filter cake with acetone-water system to obtain 189.6g of compound III, molar yield: 80% .
[0051] (2) Synthesis of Compound IV
[0052] Add 118.5g (0.5mol) of compound III, 700ml of methanol, 40.5g (0.75mol) of sodium methoxide into the reaction flask, stir at 40°C for 2 hours, follow the reaction by HPLC until the reaction of compound III is complete; quench into 2000ml of ice water, Filter and dry to obtain 99.6 g of compound IV, molar yield: 80%.
[0053] (3) Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com