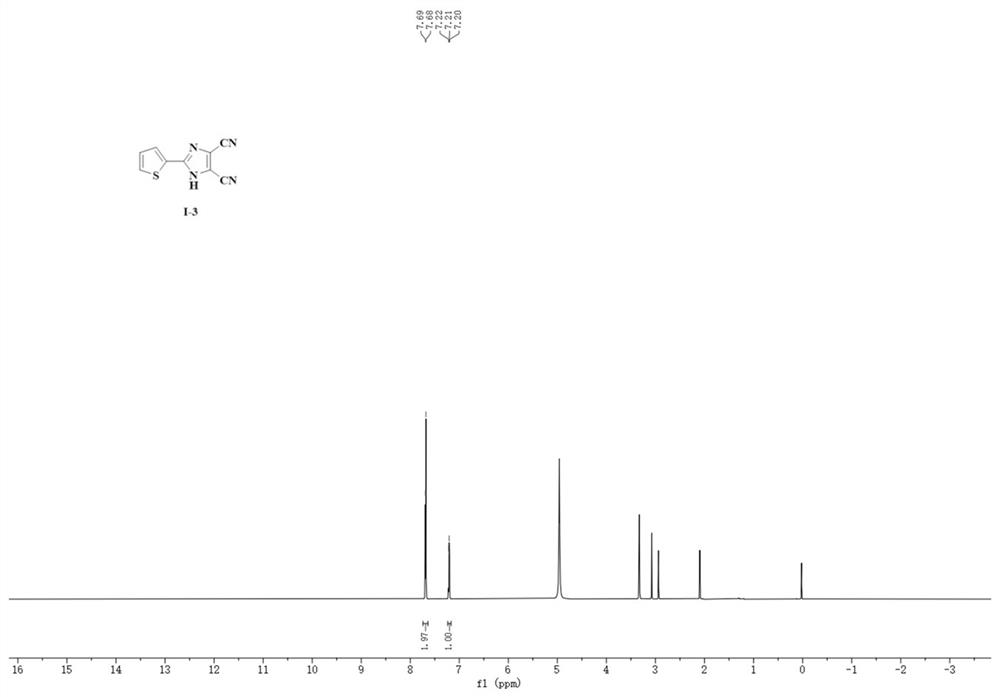
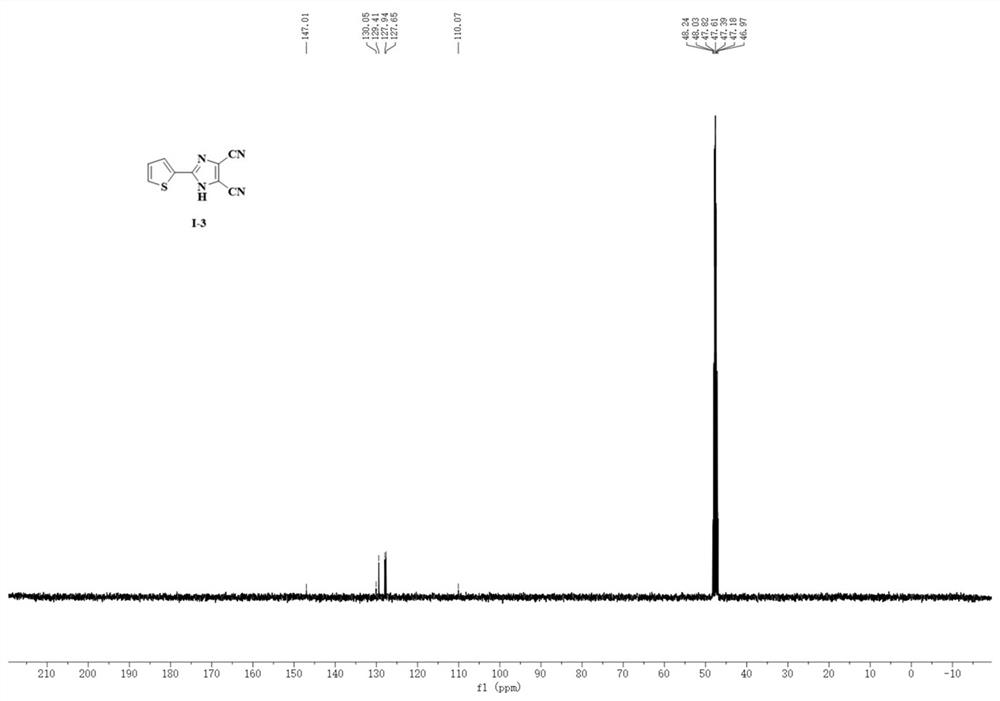
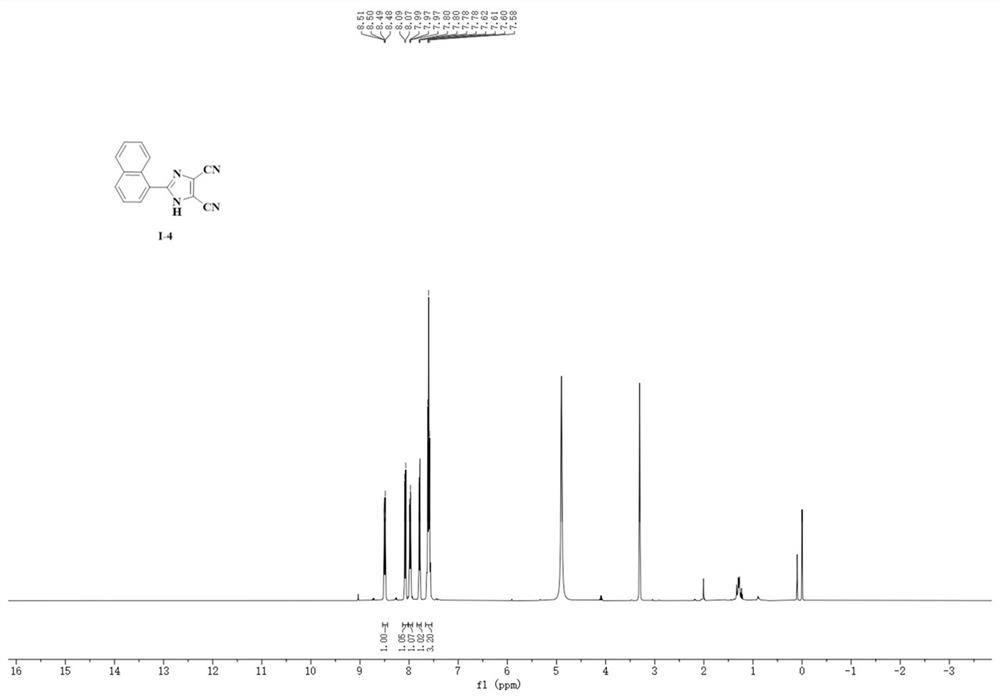
A kind of synthetic method of 4,5-dicyanoimidazole derivatives
A technology of dicyanoimidazole and synthesis method, applied in directions such as organic chemistry, can solve problems such as high cost, long reaction time, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] To the reactor was added 0.2 mmol A, 10 mg OMS-2 catalyst, and 1.5 mL DMF. After mixing uniformly, the reaction was stirred at 120°C for 2 hours, and the progress of the reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and the crude product was purified by column chromatography to obtain the target product I-1 with a yield of 22%.
Embodiment 2
[0025]
[0026] Add 0.3 mmol A, 6 mg CuO to the reactor x / OMS-2 catalyst, and then add 1.5 mL of toluene. After mixing uniformly, the reaction was stirred at 120°C for 2 hours, and the progress of the reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and the crude product was purified by column chromatography. The target product I-1 was not obtained, and the yield was 0%.
Embodiment 3
[0028]
[0029] Add 0.3 mmol A, 6 mg CuO to the reactor x / OMS-2 catalyst, and 1.5 mL of DMF was added. After mixing uniformly, the reaction was stirred at 120°C for 2 hours, and the progress of the reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and the crude product was purified by column chromatography to obtain the target product I-1 with a yield of 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com