A method for preparing a sodium iron acid for high-temperature molten salt battery-ironic acid 镧 electrode material method
A technology of high-temperature molten salt and lanthanum ferrite, applied in battery electrodes, nanotechnology for materials and surface science, secondary batteries, etc., can solve the problems of high-temperature resistance of electrode materials, complex synthesis methods, and high sintering temperatures. Achieve the effect of short drying time, simple reaction conditions and low sintering temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
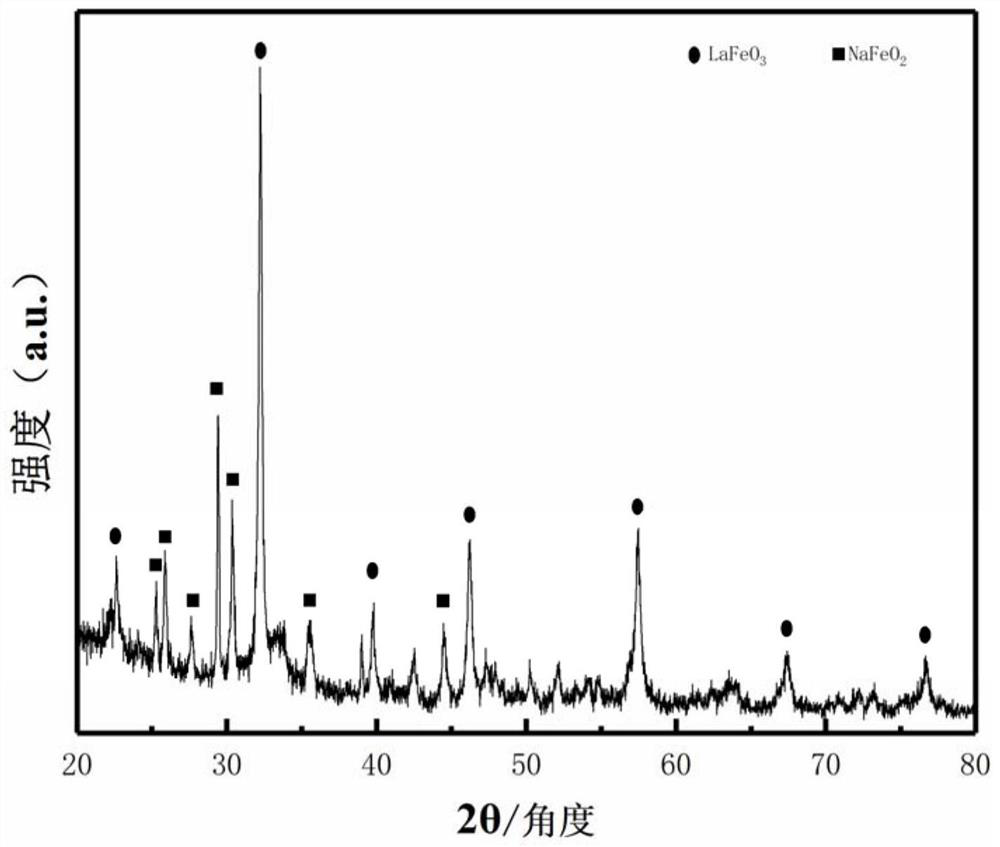
[0034] Add 0.03mol of lanthanum nitrate and 0.07mol of ferric nitrate into 200mL of deionized water, stir at 50-60°C for 2 hours until the lanthanum nitrate and ferric nitrate are completely dissolved, then add 50ml of sodium hydroxide solution at room temperature after cooling (the content of sodium hydroxide is 0.3mol) to obtain the precipitation mixture of lanthanum hydroxide and ferric hydroxide. After fully washing and centrifuging, place it in an oven and dry at 60-80°C for 24 hours to obtain a solid mixture of lanthanum and iron. Use a mortar to grind the solid mixture into powder, and place the powder sample in Aluminum oxide flakes were placed in a crucible and annealed at 400°C for 6 hours to obtain a lanthanum ferrite material. The XRD diffraction pattern, SEM scanning pattern and Coulombic efficiency pattern of the lanthanum ferrite material are as follows figure 1 , figure 2 and image 3 As shown, the sodium ferrite-lanthanum ferrite (ie sodium ferrite and lant...
Embodiment 2
[0036] Add 0.01mol of lanthanum nitrate and 0.09mol of ferric nitrate into 200mL of deionized water, stir at 50-60°C for 2 hours until the lanthanum nitrate and ferric nitrate are completely dissolved, then add 25ml of sodium hydroxide solution at room temperature after cooling (the content of sodium hydroxide is 0.3mol) to obtain the precipitation mixture of lanthanum hydroxide and ferric hydroxide. The mixed product was directly centrifuged and then dried in an oven at 60-80°C for 24 hours to obtain a solid mixture of lanthanum and iron. The solid mixture was ground into powder with a mortar, and the powder sample was Place it on an alumina sheet, put it into a crucible and anneal at 500°C for 6 hours to obtain a lanthanum ferrite material. The XRD diffraction pattern, SEM scanning pattern and Coulombic efficiency pattern of the lanthanum ferrite material are as follows Figure 4 , Figure 5 and Figure 6 As shown, the sodium ferrite-lanthanum ferrite heterostructure nanoe...
Embodiment 3
[0038] Add 0.07mol of lanthanum nitrate and 0.03mol of ferric nitrate into 200mL of deionized water, stir at 50-60°C for 2 hours until the lanthanum nitrate and ferric nitrate are completely dissolved, then add 25ml of sodium hydroxide solution at room temperature after cooling (the content of sodium hydroxide is 0.3mol) to obtain the precipitation mixture of lanthanum hydroxide and ferric hydroxide. The mixed product was directly centrifuged and then dried in an oven at 60-80°C for 24 hours to obtain a solid mixture of lanthanum and iron. The solid mixture was ground into powder with a mortar, and the powder sample was Place it on an alumina sheet, put it into a crucible and anneal at 500°C for 6 hours to obtain a lanthanum ferrite material. The XRD diffraction pattern, SEM scanning pattern and Coulombic efficiency pattern of the lanthanum ferrite material are as follows Figure 7 , Figure 8 and Figure 9 As shown, the sodium ferrite-lanthanum ferrite heterostructure nanoe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com