Preparation method and application of a class of continuon-type macrocyclic diterpenoids
A technology of sacron-type and compound, which is applied in the field of preparation of continu-type macrocyclic diterpene compounds, can solve problems such as toxicity, and achieve the effect of improving the degree of liver damage and good PXR agonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of Compounds 1 to 5
[0049]
[0050] The seeds (8kg) of S. japonica were soaked with 95% ethanol (75L×3) to obtain 817.2g crude extract. The extract was dispersed in water (3 L) and extracted with petroleum ether, ethyl acetate and n-butanol in sequence. The ethyl acetate fractional extract (352.8 g) was subjected to gradient elution (MeOH / H) with D101 macroporous adsorption resin 2 O, 6:4→10:0) separated into three parts (Fr.I-III). The Fr.II part (215.6 g) was separated into four parts (Fr.IIA-IID) by normal phase silica gel column chromatography (PE / EtOAc, 50:1→1:1). Fr.IIC segment (2.9g) by reversed phase C 18 Silica gel column chromatography (MeOH / H 2 O,6:4→10:0) separated into five parts (Fr.IIC 1 -IIC 5 ), where Fr.IIC 1 A fraction (132.2 mg) was subjected to normal phase silica gel column chromatography (CH 2 Cl 2 / MeOH, 100:1→0:1) gradient elution separation and semi-preparative chiral separation HPLC (MeCN / H 2 O, 75 / 25, 3mL...
Embodiment 2
[0062] Example 2: Preparation of Compound 8
[0063]
[0064] Fr.IIC in Example 1 2 Fragment (1.435g) was separated by Sephadex LH-20 gel column (MeOH) and then by normal phase silica gel column chromatography (PE / EtOAc, 10:1→0:1) to obtain three segments (Fr.IIC). 2a -IIC 2c ). Fr.IIC 2a (207.2 mg) by normal phase silica gel column chromatography (PE / EtOAc, 15:1→0:1) followed by semi-preparative chiral separation by HPLC (MeCN / H 2 O, 70 / 30, 3 mL / min) to obtain compound 8 (67.3 mg, t R 8.9min).
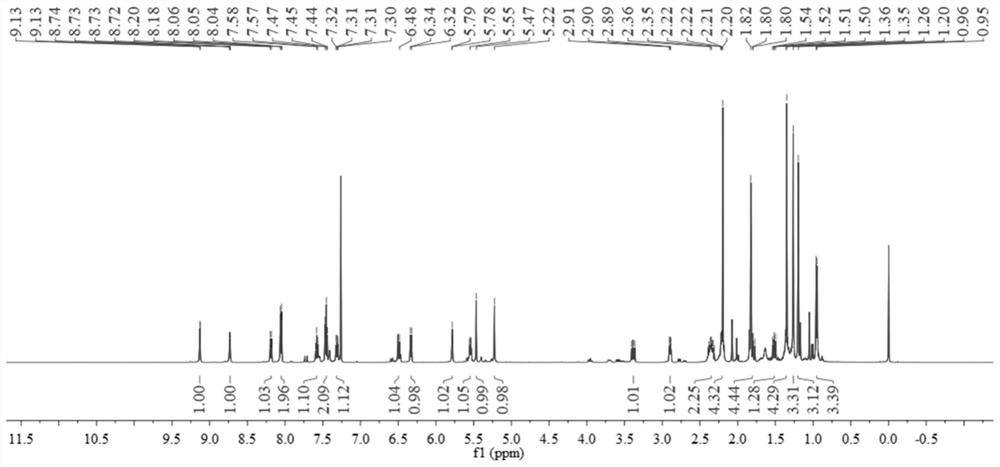
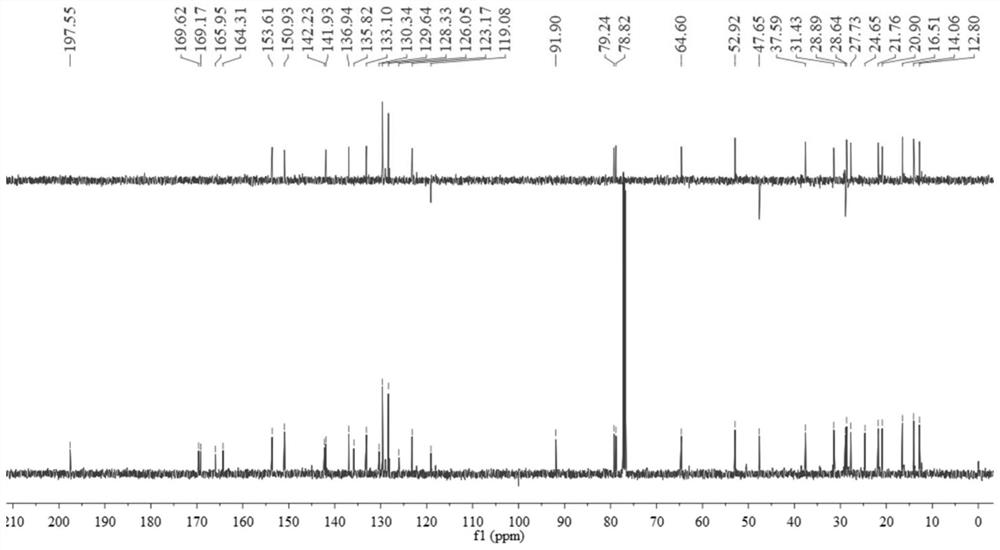
[0065] figure 1 and figure 2 They are the H NMR, C and DEPT135 spectra of compound 8, respectively. Its spectral data is consistent with the known compound Euphorbia Factor L 9 Consistent.
Embodiment 3
[0066] Example 3: Preparation of Compound 17
[0067]
[0068]Compound 15 (30 mg, 0.047 mmol) was dissolved in 2 mL of methanol, 1% sodium hydroxide was added, and the reaction was carried out at room temperature for 1 hour. The reaction solution was quenched with 5 mL of water, and extracted with ethyl acetate (5 mL×3). The organic layer was concentrated and spun dry, and purified by silica gel column chromatography CC (PE / EtOAc, 10:1) to give compound 17 (15.3 mg).
[0069] Its spectral data are consistent with literature reports, so its structure is determined.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com