Coordinated molybdenum-oxygen heterocyclic catalyst, and preparation method and application thereof
A technology of catalyst and oxygen heterocycle, applied in the field of coordination molybdenum oxygen heterocycle catalyst and its preparation, can solve the problem of low selectivity of propylene oxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] One coordinating oxetane molybdenum catalyst, the following chemical structure:
[0032] Where L 1 for
[0033] The method for preparing a coordination oxetane molybdenum catalyst, comprising the steps of:
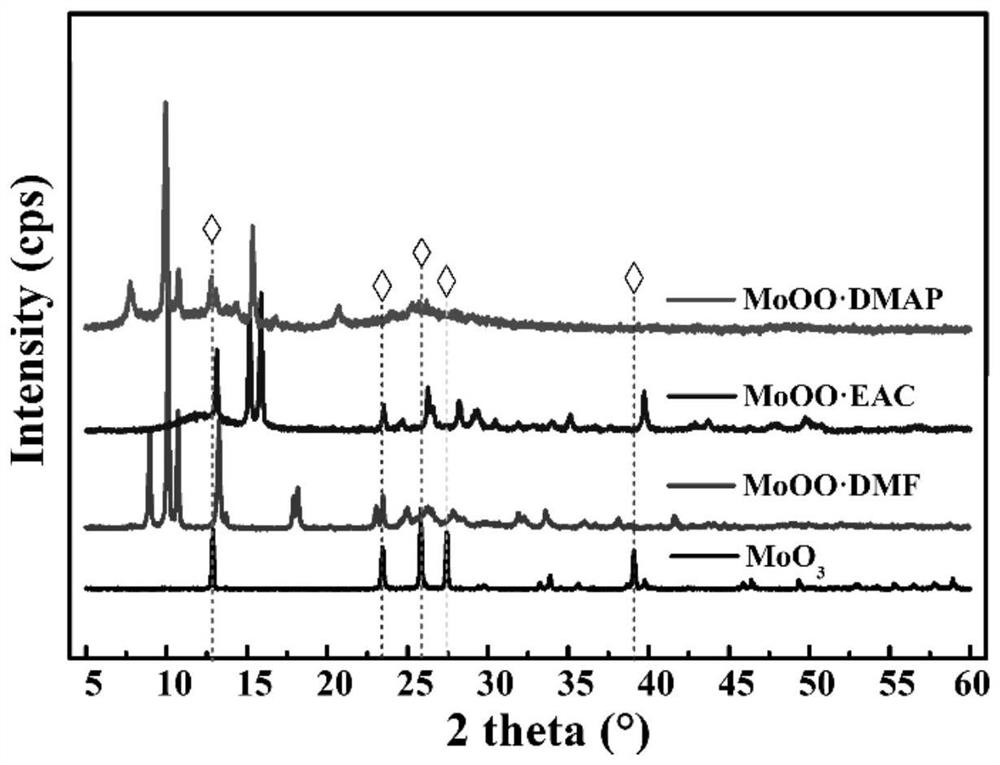
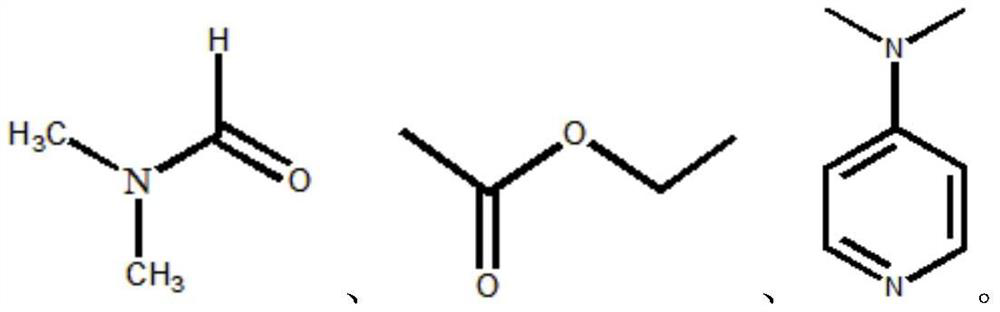
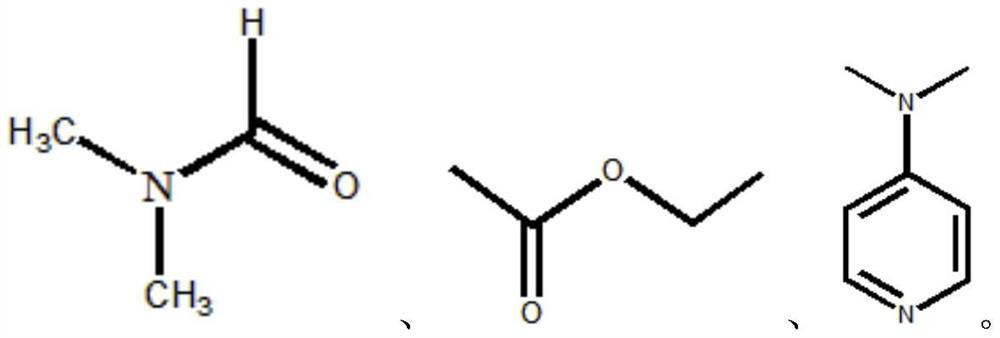
[0034] . S1 3.5g of molybdenum trioxide was added to the round bottom flask, was slowly added under stirring to 10ml 30% hydrogen peroxide solution, stirred at reflux for 10h at 50 deg.] C to form a clear solution A; ethyl acetate formulation taken 2mol a solution B;
[0035] S2. Under stirring, solution A to solution B obtained was filtered solution C, the reaction solution was refluxed for 3h C continues placed in the refrigerator at 8 ℃ refrigerator 5d 50 ℃, until crystals precipitated crystals were filtered, washed drying, to give ligand oxetanyl molybdenum catalyst MoOO · EAC.
Embodiment 2
[0037] One coordinating oxetane molybdenum catalyst, the following chemical structure:
[0038] Where L 1 for
[0039] The method for preparing a coordination oxetane molybdenum catalyst, comprising the steps of:
[0040] . S1 5g of molybdenum trioxide was added to the round bottom flask, was slowly added under stirring to 20ml 30% hydrogen peroxide solution, stirred at reflux for 15h at 40 ℃ A to form a clear solution; take 3mol N, N- two dimethylformamide formulated as a solution B;
[0041] S2. Under stirring, solution A to solution B obtained was filtered solution C, the reaction solution was refluxed for 5h C continues placed in the refrigerator at 2 ℃ refrigerated 6d 40 ℃, until crystals precipitated crystals were filtered, washed drying, to give ligand oxetanyl molybdenum catalyst MoOO · DMF.
Embodiment 3
[0043] One coordinating oxetane molybdenum catalyst, the following chemical structure:
[0044] Where L 1 for
[0045] The method for preparing a coordination oxetane molybdenum catalyst, comprising the steps of:
[0046] . S1 6g molybdenum trioxide was added to the round bottom flask, was slowly added under stirring to 12ml 30% hydrogen peroxide solution, stirred at reflux for 10h at 45 ℃ A to form a clear solution; take 2mol 4- dimethylaminopyridine pyridine formulated as a solution B;
[0047] S2. Under stirring, solution A to solution B obtained was filtered solution C, the reaction solution was continued to reflux for 4h C was placed in a refrigerator at 5 ℃ refrigerated 2d 45 ℃, until crystals precipitated crystals were filtered, washed drying, to give ligand oxetanyl molybdenum catalyst MoOO · DMAP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com