Prophylactic or therapeutic drug for benign tumor
A benign tumor and pharmaceutical technology, applied in the field of the composition for preventing or treating familial adenomatous polyposis, the drug for preventing or treating benign tumors, and can solve the problems of weak effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0663] (Example 1: Expression of WT1 protein in adenoma of familial adenomatous polyposis)
[0664] This example shows that WT1 protein is expressed in adenomas of human patients with familial adenomatous polyposis.
[0665] (experimental method)
[0666] The small and large intestines of a human patient with familial adenomatous polyposis were formalin-fixed and paraffin-embedded, and paraffin blocks were sectioned to a thickness of 3 μm. To reduce endogenous peroxidase reactions, paraffin sections were washed with 3% HO 2 o 2Solution processed, then deparaffinized in xylene, and after stepwise rehydration with ethanol, for antigen retrieval, in 10 mM Tris EDTA buffer (10 mM Tris, 1 mM EDTA, pH 9.0) in a 70°C oil bath Heat for 25 minutes and heat in an oil bath at 110° C. for 25 minutes and cool at room temperature for 3 hours. The treated sections were reacted overnight at 4°C with anti-WT1 6FH2 mouse monoclonal antibody (Dako Cytomation, Inc., Carpinteria, CA) diluted 1...
Embodiment 2
[0671] (Embodiment 2: in APC Min / + Expression of WT1 protein in mice)
[0672] This example shows that WT1 protein in APC Min / + Expressed in small intestinal adenomas of mice, a model mouse for familial adenomatous polyposis.
[0673] (experimental method)
[0674] 20 weeks old APC Min / + The small and large intestines of (C57BL / 6J) mice were formalin-fixed, and paraffin-embedded, and paraffin blocks were sectioned to a thickness of 3 μm. Paraffin sections were deparaffinized with xylene and, after stepwise rehydration with ethanol, microwaved for 15 min in 10 mM citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for antigen retrieval . The treated sections were reacted overnight at 4°C with anti-WT1 6FH2 mouse monoclonal antibody (Dako Cytomation, Inc., Carpinteria, CA) diluted 100-fold in PBS containing goat serum. Then, the sections were reacted with a biotinylated anti-mouse IgG antibody (Vector Labs., Burlingame, CA) diluted 100-fold in PBS containing goa...
Embodiment 3
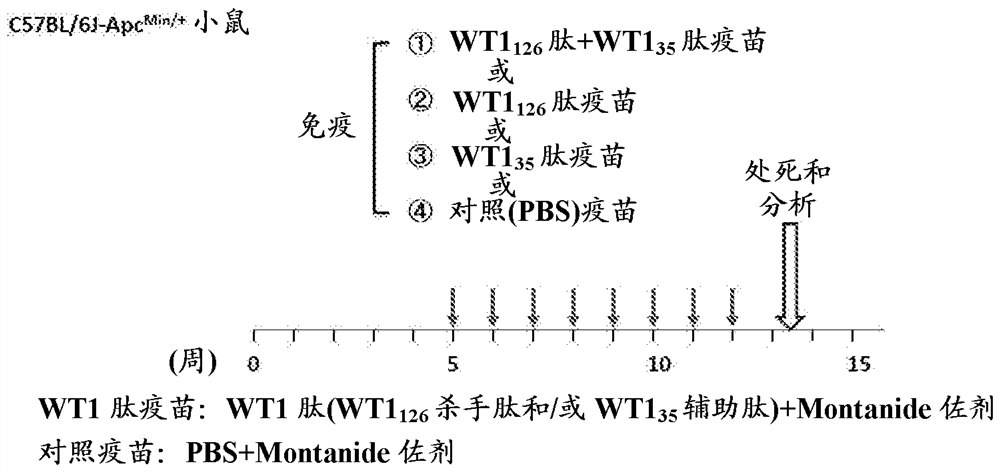
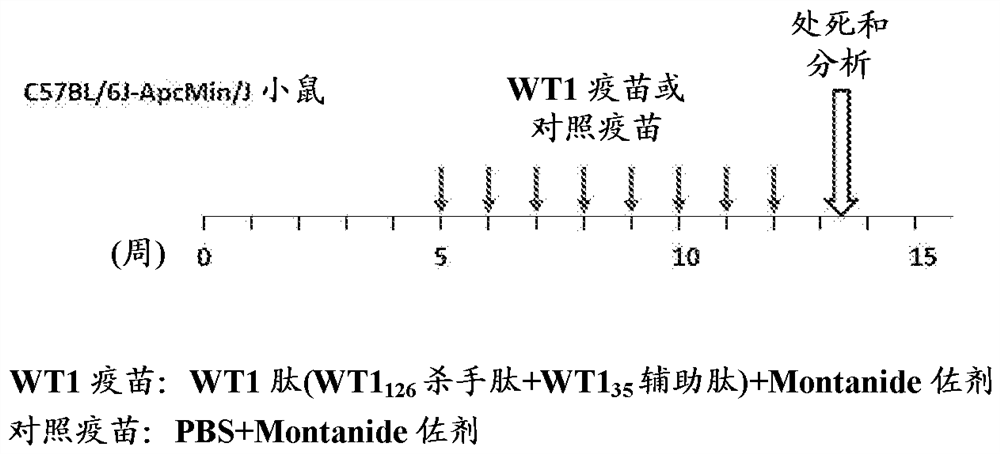
[0677] (Example 3: Administering WT1 Peptide Vaccine to Familial Adenomatous Polyposis Model Mice)
[0678] This example shows the administration of WT1 peptide vaccine to APC Min / + Experimental dosing regimen for mice.
[0679] (experimental method)
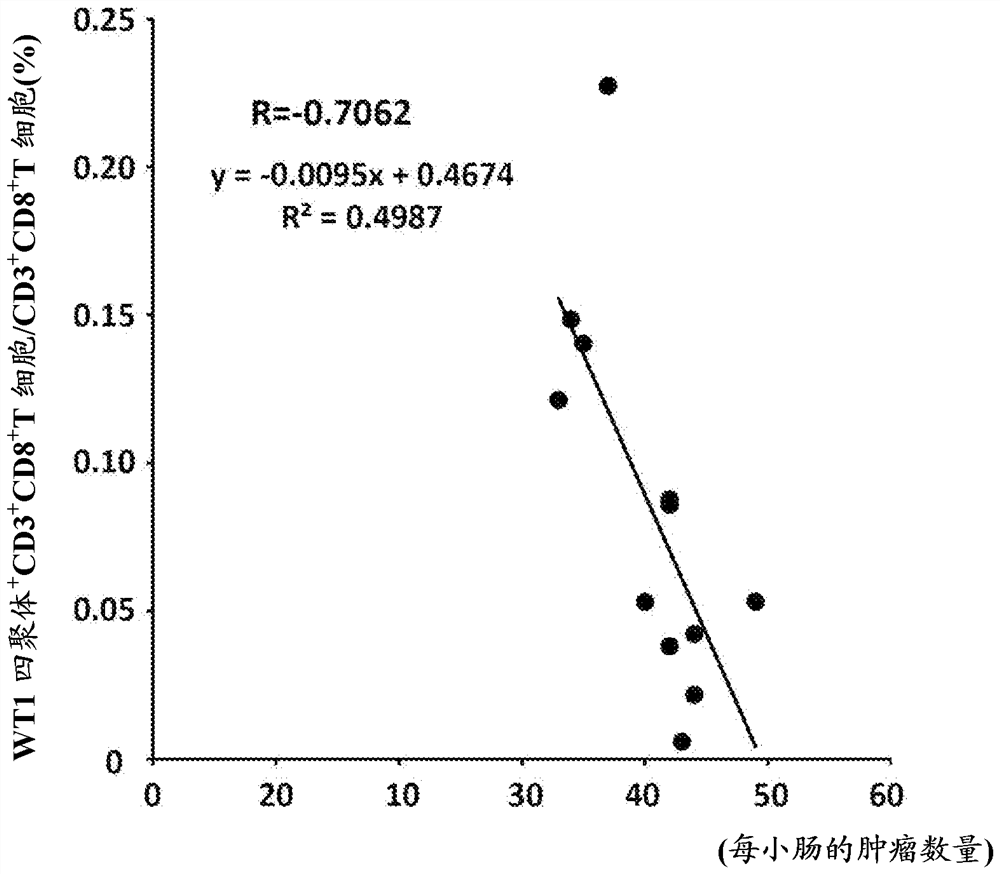
[0680] The dosing regimen for the WT1 peptide vaccine is shown in Figure 4 . 100 μg of WT1 126-134 Killer peptide and 45 μg of WT1 35-52 The mixture of helper peptide or PBS was emulsified with IFA Montanide ISA51 adjuvant to obtain WT1 peptide vaccine or control vaccine. WT1 peptide vaccine or control vaccine administered intradermally to 4- to 5-week-old APCs Min / + The flank of the mouse. Immunizations were started from the 5th week of life and performed 8 times at a frequency of once a week. Immunized mice were euthanized 10 days after the last immunization for further analysis. Administration of the WT1 peptide vaccine did not cause organ damage, as shown in Table 3 below.
[0681] table 3
[0682]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com