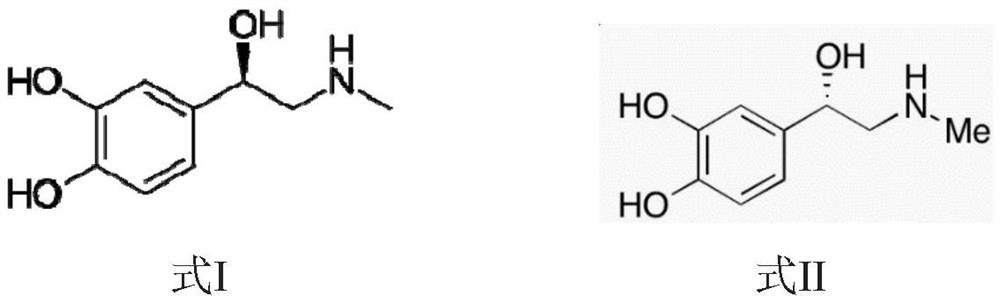
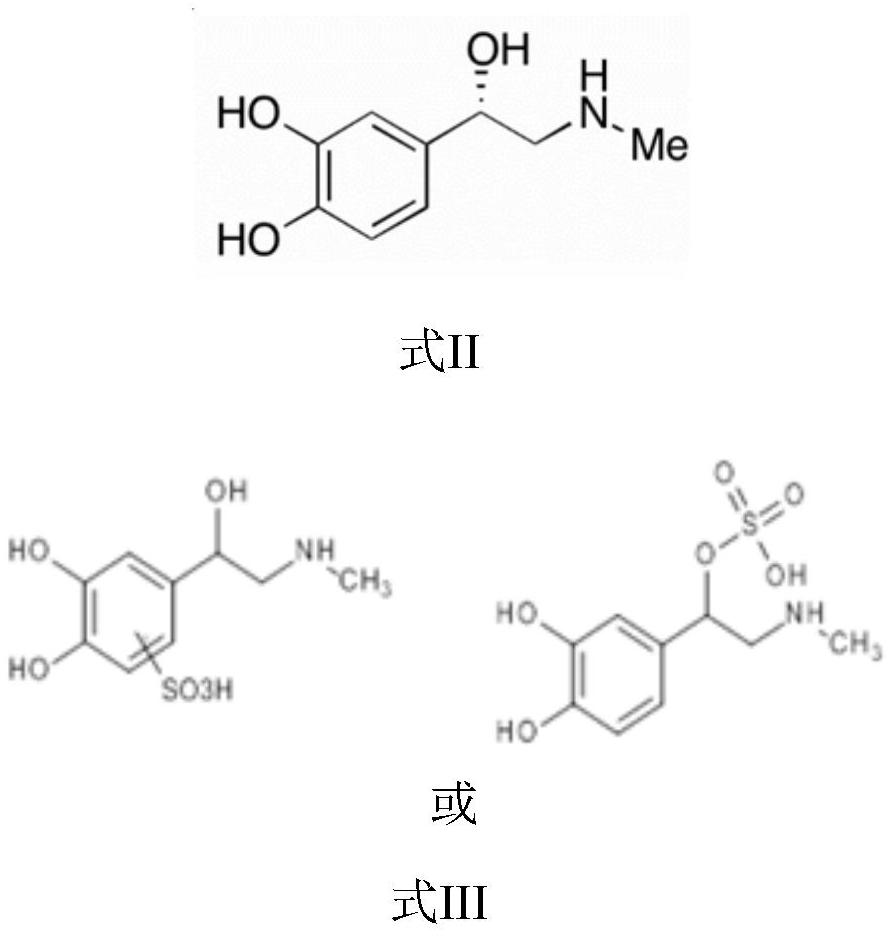
Stable aqueous injectable solution of epinephrine
A technology of epinephrine and epinephrine base, applied in the field of injectable epinephrine aqueous solution, which can solve the problems of no epinephrine and low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
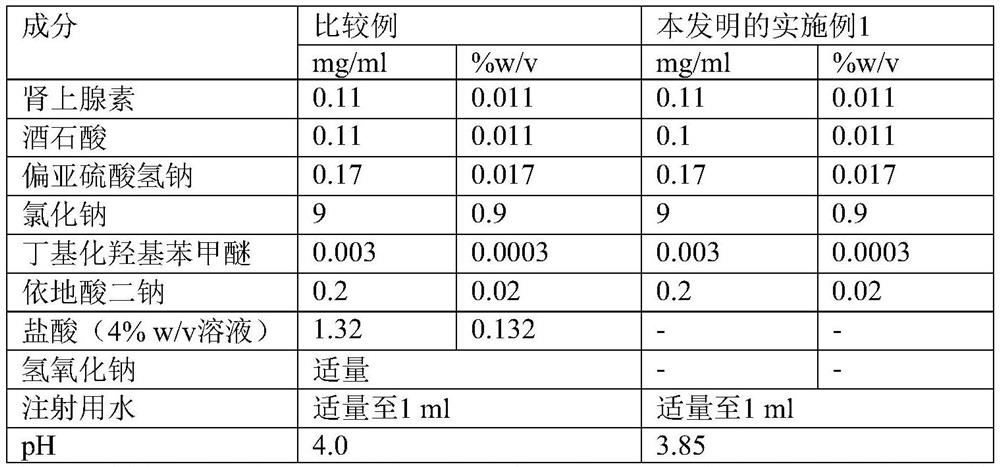
[0034] Preparation method-comparative example: Water for injection was placed in a glass container, and nitrogen gas was continuously purged into it to achieve oxygen dissolution and maintain the dissolved oxygen level below 1 ppm. The solution was cooled to 2-8°C. Add weighed amounts of butylated hydroxyanisole, edetate disodium, sodium chloride, epinephrine, hydrochloric acid, and sodium metabisulfite to the above container while stirring and continuously purging nitrogen. The pH was adjusted to about 4.0 using a solution of 1% w / v sodium hydroxide and hydrochloric acid with a continuous nitrogen purge. Make up the volume with water for injection. Filter the solution through a 0.2 micron PVDF capsule filter. Fill 10ml of filtered solution into a cartridge with a standard fill volume of 10.5ml. The filled cartridge is plugged with a plunger. Place the prefilled syringe in a polyethylene bag. Place the polyethylene bag containing the prefilled syringe in a cor...
Embodiment 2
[0037] The stabilized injectable aqueous epinephrine solutions of Comparative Example and Example 1 filled in prefilled syringes were placed in polyethylene bags which were placed in corrugated boxes and kept at 40°C / 75% relative humidity store. The chemical stability of the solutions was assessed at 1M, 3M and 6M time points. These analytes were measured by standard analytical procedures. Table 2 below presents the results.
Embodiment 3
[0039] The stable injectable adrenaline aqueous solution of Comparative Example and Example 1 filled in prefilled syringes was packaged in an aluminum bag containing an oxygen scavenger and placed in a corrugated box and kept at 40°C / 75% Store under relative humidity. The chemical stability of the solutions was assessed at 1M, 3M and 6M time points. These analytes were measured by standard analytical procedures. Table 2 below presents the results.
[0040] Table 2: Stability data for comparative examples and examples of the invention
[0041]
[0042] M-*month
[0043] From the stability data, it can be concluded that in the case of the solution according to the invention, the content of impurities (sulphate impurities and D-epinephrine) changed slightly, while in the comparative example, on storage, the impurities content significantly increased. In the comparative example, it was found that the presence of mineral acid caused an increase in known impurities such as D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com