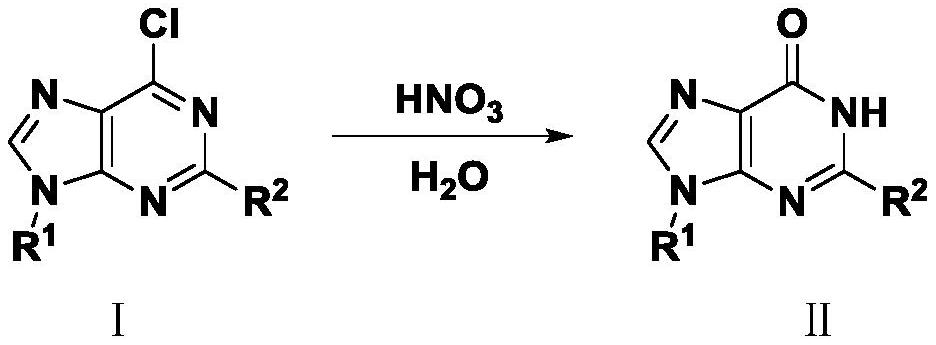
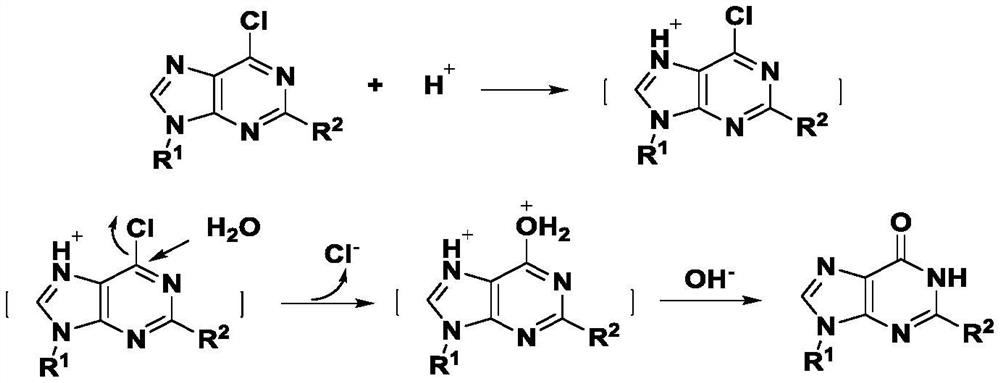
Synthesis method of hypoxanthine derivative under catalysis of nitric acid
A technology of nitric acid catalyzing hypoxanthine and a synthesis method, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, etc., can solve the problems of corrosion equipment, harsh reaction conditions, poor substrate adaptability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a reaction flask, add 6-chloropurine (0.154 g, 1 mmol) to acetonitrile (4 mL) and H 2 O (1mL), add 68% nitric acid (0.13μL, 0.02mmol), heat to 80℃, react for 2h, add saturated NaHCO 3 The solution was neutralized, the reaction solution was transferred to a concentrator, and concentrated under reduced pressure to obtain 0.125 g of hypoxanthine with a yield of 92%.
Embodiment 2
[0030] In a reaction flask, add 6-chloropurine (0.154 g, 1 mmol) to toluene (4 mL) and H 2 O (1mL), add 68% nitric acid (0.13μL, 0.02mmol), heat to 80℃, react for 2h, add saturated NaHCO 3 The solution was neutralized, the reaction solution was transferred to a concentrator, and concentrated under reduced pressure to obtain 0.116 g of hypoxanthine with a yield of 85%.
Embodiment 3
[0032] In a reaction flask, add 6-chloropurine (0.154 g, 1 mmol) to chloroform (4 mL) and H 2 O (1 mL), add 68% nitric acid (0.13 μL, 0.02 mmol), seal the reaction bottle, heat to 80 ° C for 4 h, add saturated NaHCO 3 The solution was neutralized, and the reaction solution was transferred to a concentration device, and concentrated under reduced pressure to obtain 0.103 g of hypoxanthine with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com