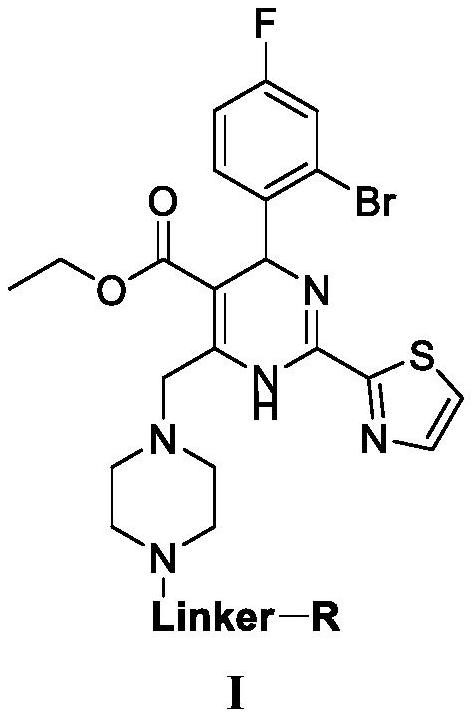
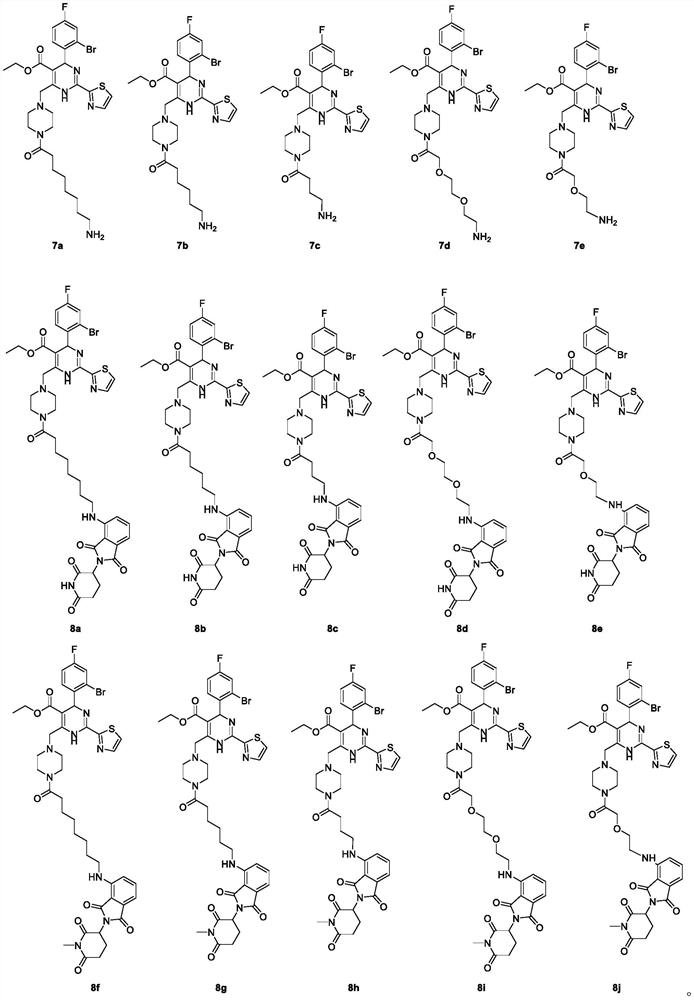
Dihydropyrimidine-pomalidomide conjugate as well as preparation method and application thereof
A technology of pomalidomide and dihydropyrimidine, applied in the field of anti-HBV drugs, dihydropyrimidine-pomalidomide conjugates and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
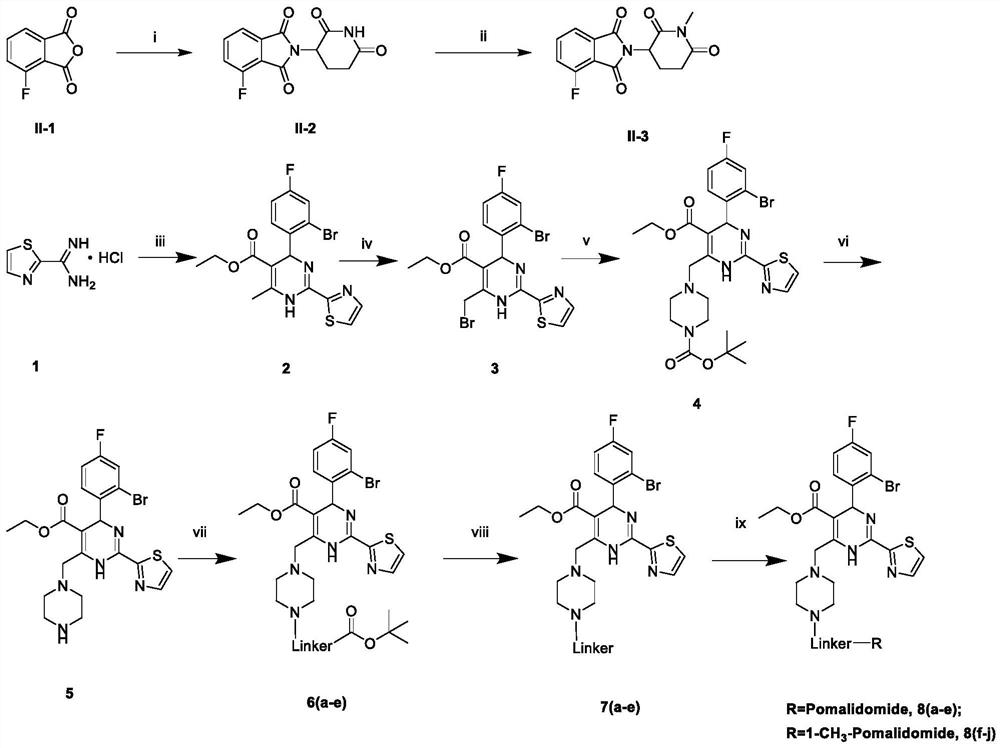
[0052] Embodiment 1. Preparation of compound II-2
[0053] Dissolve 3-fluorophthalic anhydride (200mg, 1.2mmol), 3-amino-2,6-piperidinedione hydrochloride (259mg, 1.2mmol) sodium acetate (118mg, 1.44mmol) in 20ml of acetic acid, 120°C Reflux 10h. After the reaction, the solvent was spin-dried to obtain a large amount of black solid, which was dissolved by adding a large amount of methanol, and then added to silica gel to mix the sample and put it on the column, separated by flash column chromatography to obtain II-2.
[0054] White solid product, yield 58%; 1 H NMR (400MHz, DMSO-d 6 )δ11.15 (s, 1H, CONHCO), 7.95 (q, J = 7.4Hz, 1H, Ph-H), 7.83–7.66 (m, 2H, Ph-H), 5.16 (dd, J = 12.8, 5.1 Hz,1H,COCHN),2.97–2.82(m,1H,CH 2 ),2.68–2.52(m,1H,CH 2 ),2.18–1.96(m,2H,CH 2 ); EI-MS: 275.06[M-H] - ;C 13 h 9 FN 2 o 4 [276.05].
Embodiment 2
[0055] Embodiment 2. Preparation of compound II-3
[0056] Add II-2 (100.00mg, 0.36mmol) and potassium carbonate (50mg, 0.36mmol) into the solvent N,N-dimethylformamide 5ml, and stir at room temperature, add iodomethane dropwise, and stir at room temperature for 24h. After the reaction, spin the solvent to dry, add water (20ml) and dichloromethane (20ml*2) for extraction, combine the organic phases, extract once with saturated sodium chloride, dry with anhydrous magnesium sulfate, and filter. The organic phase was added to silica gel to mix the sample, and separated by flash column chromatography to obtain II-3.
[0057] White solid product, yield 46%; 1 H NMR (400MHz, DMSO-d 6 )δ7.96 (tdt, J=7.4, 4.7, 2.3Hz, 1H, Ph-H), 7.83–7.70 (m, 2H, Ph-H), 5.30–5.12 (m, 1H, COCHN), 3.08–2.85 (m,3H,CH 3 ),2.85–2.73(m,1H,CH 2 ),2.66–2.51(m,1H,CH 2 ), 2.08 (tdd, J=12.7, 7.5, 4.2Hz, 1H, CH 2 ); EI-MS: 290.25[M-H] - ;C 14 h 11 FN 2 o 4 [290.07].
Embodiment 3
[0058] Embodiment 3. Preparation of Compound 2
[0059] Weigh 2-thiazole formamidine hydrochloride (1.0g, 6.11mmol), 2-bromo-4-fluorobenzaldehyde (1.86g, 9.16mmol) and sodium acetate (1.0g, 1.22mmol) were dissolved in absolute ethanol ( 100 mL), ethyl acetoacetate (1.2 mL, 9.20 mmol) was added under stirring at room temperature, and ethanol was refluxed at 80° C. for 8 h; after the reaction was completed, it was filtered to remove salts. The mother liquor was cooled to room temperature, and yellow crystals (I-2) were precipitated. Remove absolute ethanol from the remaining mother liquor under reduced pressure, add water (60mL), extract with ethyl acetate (25mL×3), collect and combine the organic phases, extract once with saturated sodium chloride (25mL), and use anhydrous magnesium sulfate for the organic phase dry. Filter, add 200 mesh silica gel, mix the sample, separate by flash column chromatography, and recrystallize to obtain compound 2. 0.75 g of yellow powder was ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com