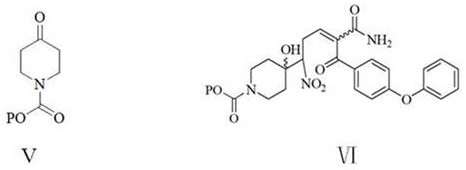
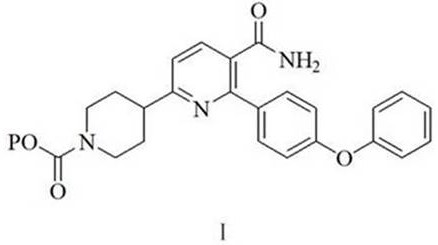
A kind of preparation method of 2-(4-phenoxyphenyl)-6-(n-substituted oxycarbonyl piperidine-4-) base nicotinamide
A technology of phenoxyphenyl and base carbonyl piperidine is applied in the field of preparation of 2--6-yl nicotinamide, and achieves the effects of low cost, easy operation of technological process, and cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
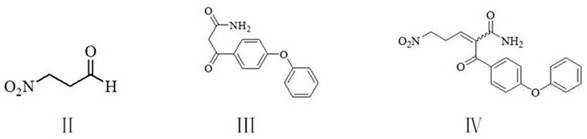
[0058] Example 1: Preparation of 2-(4-phenoxybenzoyl)-5-nitro-n-pent-2-enamide (IV)
[0059] To a 500-mL four-necked flask equipped with a stirrer, a thermometer, a water separator and a reflux condenser, add 250 g of cyclohexane, 20.6 g (0.2 mol) of 3-nitropropanal, 51.0 g (0.2 mol) of 3-( 4-phenoxyphenyl)-3-oxopropionamide, 0.6 g of p-toluenesulfonic acid, reflux with water at 80-82°C for 3 hours with stirring, until the water is completely carried. The solvent was recovered by distillation under reduced pressure at 40°C, 200 g of 90% methanol aqueous solution was added to the residue, recrystallized by heating, filtered and dried to obtain 63.0 g of 2-(4-phenoxybenzoyl)-5-nitro n-Pent-2-enamide (IV), yield 92.6%, liquid phase purity 99.9%.
Embodiment 2
[0060] Example 2: Preparation of 2-(4-phenoxybenzoyl)-5-nitro-n-pent-2-enamide (IV)
[0061] To a 500 ml four-necked flask equipped with stirring, a thermometer and a reflux condenser, add 250 g of ethanol, 20.6 g (0.2 mol) of 3-nitropropionaldehyde, 51.0 g (0.2 mol) of 3-(4-phenoxybenzene) yl)-3-oxopropionamide, 0.5 g of 98% sulfuric acid, and the reaction was stirred at 60-65 °C for 3 hours. Cool to 10-15°C, filter and dry to obtain 61.5 g of 2-(4-phenoxybenzoyl)-5-nitro-n-pent-2-enamide (IV), yield 90.4%, liquid phase purity 99.7%.
Embodiment 3
[0062] Example 3: Preparation of 2-(4-phenoxybenzoyl)-5-nitro-n-pent-2-enamide (IV)
[0063] To a 500-mL four-necked flask equipped with a stirrer, a thermometer, a water separator and a reflux condenser, add 250 g of 2-methyltetrahydrofuran, 20.6 g (0.2 mol) of 3-nitropropanal, 51.0 g (0.2 mol) of 3 -(4-phenoxyphenyl)-3-oxopropionamide, 0.6 g of p-toluenesulfonic acid, reflux with water for 3 hours under stirring at 75-80° C. until the water is completely carried. The solvent was recovered by distillation under reduced pressure at 40°C, 200 g of 90% methanol aqueous solution was added to the residue, recrystallized by heating, filtered and dried to obtain 62.1 g of 2-(4-phenoxybenzoyl)-5-nitro n-Pent-2-enamide (IV), yield 91.3%, liquid phase purity 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com