A kind of preparation method of 2-(4-phenoxyphenyl)-6-(piperidin-4-) base nicotinamide
A technology of phenoxyphenyl, nicotinamide, which is applied in the field of medicine and chemical industry, and can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
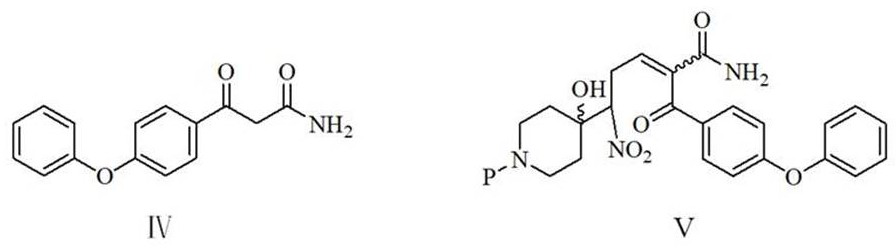
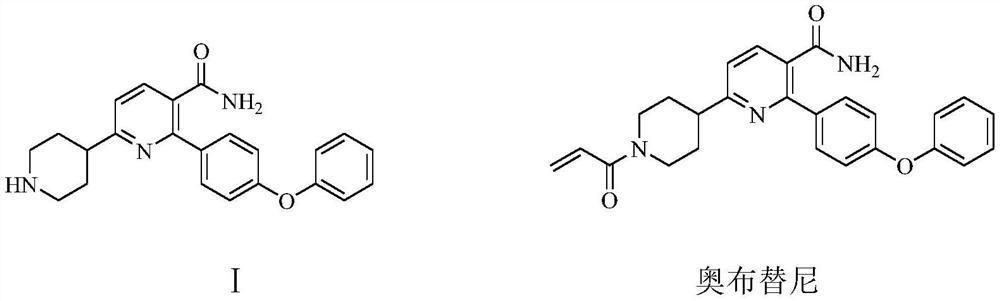
[0051] Example 1: Preparation of 2-(4-phenoxyphenyl)-6-(piperidin-4-)ylnicotinamide (I)
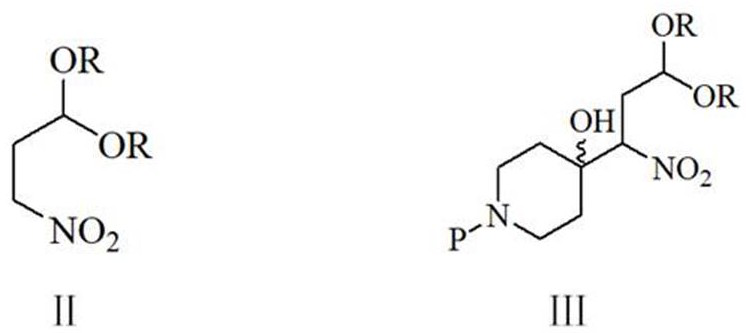
[0052] To a 1000 ml four-necked flask equipped with a stirring and thermometer, add 100 g of water, 200 g of methanol, 29.8 g (0.2 mol) of 3,3-dimethoxy-1-nitropropane, 26 g (0.26 mol) of 40 % sodium hydroxide, cooled, keeping the temperature at 0-5 ℃, dropwise added a solution of 41.5 g (0.22 mol) N-benzylpiperidin-4-one and 100 g methanol, the dropwise addition was completed in about 1 hour, after that, 10 The reaction was stirred at -15°C for 3 hours. 25 g of ammonium chloride, 51.5 g (0.202 mol) of 3-(4-phenoxyphenyl)-3-oxo-propionamide were added, and the reaction was stirred at 40-45° C. for 4 hours. The obtained reaction solution was transferred to a 1000 ml stainless steel autoclave, 0.8 g of 5% palladium carbon and 0.15 g of triphenylphosphine were added, and after nitrogen replacement 3 times, the pressure was filled with hydrogen to 0.4-0.5 MPa, and 30-35 ℃ of catalytic hydrog...
Embodiment 2
[0056] Example 2: Preparation of 2-(4-phenoxyphenyl)-6-(piperidin-4-)ylnicotinamide (I)
[0057] To a 1000 ml four-necked flask equipped with a stirring thermometer, add 100 g of water, 200 g of methanol, 35.4 g (0.2 mol) of 3,3-diethoxy-1-nitropropane, 16.8 g (0.3 mol) of hydrogen Potassium oxide, cooled, keeping the temperature at 0-5 ℃, dropwise added a solution of 41.5 g (0.22 mol) N-benzylpiperidin-4-one and 80 g methanol, the dropwise addition was completed in about 1.0 hours, after that, 10-15 The reaction was stirred for 3 hours. 30 g of ammonium chloride, 51.5 g (0.202 mole) of 3-(4-phenoxyphenyl)-3-oxo-propionamide were added, and the reaction was stirred at 40-45° C. for 4 hours. The obtained reaction solution was transferred to a 1000-milliliter stainless steel autoclave, 0.8 g of 5% palladium carbon and 0.15 g of triphenylphosphine were added, and after nitrogen replacement for 3 times, the pressure was filled with hydrogen to 0.4-0.5 MPa, and catalytic hydrogena...
Embodiment 3
[0058] Example 3: Preparation of 2-(4-phenoxyphenyl)-6-(piperidin-4-)ylnicotinamide (I)
[0059] To a 1000 ml four-necked flask equipped with a stirring thermometer, add 100 g of water, 200 g of methanol, 35.4 g (0.2 mol) of 3,3-diethoxy-1-nitropropane, 16.8 g (0.3 mol) of hydrogen Potassium oxide, cooled, keeping the temperature at 0-5 °C, dropwise added a solution of 48.2 g (0.22 mol) N-(4-p-methoxybenzyl)piperidin-4-one and 100 g methanol, about 1.0 hours dropwise After the addition was complete, the reaction was stirred at 15-20°C for 3 hours. 30 g of ammonium chloride and 51.5 g (0.202 mole) of 3-(4-phenoxyphenyl)-3-oxo-propionamide were added, and the reaction was stirred at 40-45° C. for 4 hours. The obtained reaction solution was transferred to a 1000-milliliter stainless steel autoclave, 0.8 g of 5% palladium carbon and 0.15 g of 4-dimethylaminopyridine were added, and after 3 times of nitrogen replacement, the pressure was filled with hydrogen to 0.4-0.5 MPa, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com