Bispecific antibody targeting NKG2A and PD-L1 and application
A multispecific antibody and antibody technology, applied in the field of antibody drugs and tumor therapeutic antibodies, can solve the problems of insufficient antigen affinity and poor therapeutic effect, and achieve the effect of improving affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1. Expression Analysis of Anti-NKG2A Specific Antibody Z270 Antibody
[0070] The coding genes of NKG2A (genebank: AF461812.1, 157aa-233aa) and CD94 (genebank: AB009597.1, 4aa-148aa) were connected through the GGSGGS coding gene to synthesize the coding gene of NKG2A-CD94 (SEQ ID NO.1), and Further cloned into eukaryotic transient expression vectors with mFc tag at the N-terminal by enzyme digestion method, and transferred into Escherichia coli to amplify, isolated and obtained mFc-NKG2A-CD94 expression plasmid, and transfected according to the transfection reagent 293fectin (Cat: 12347019 , Gibco), the plasmid was transferred into HEK293 cells for recombinant expression. 5-6 days after the cells were transfected, the culture supernatant was taken, and the expression supernatant was purified using a ProA affinity chromatography column to obtain pure mFc-NKG2A-CD94 recombinant protein.
[0071] Obtain the light and heavy chain amino acid sequences of the antibod...
Embodiment 2
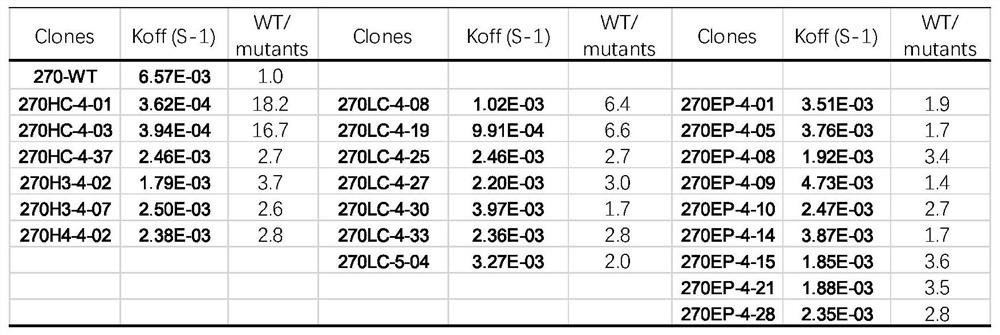
[0074] Example 2. Affinity maturation of anti-NKG2a antibody hum270
[0075] Using yeast display technology, the anti-NKG2a antibody hum270 heavy chain variable region and 6 CDR region amino acids of the light chain variable region were randomly mutated to build a library, and candidate antibody molecules with higher affinity were screened.
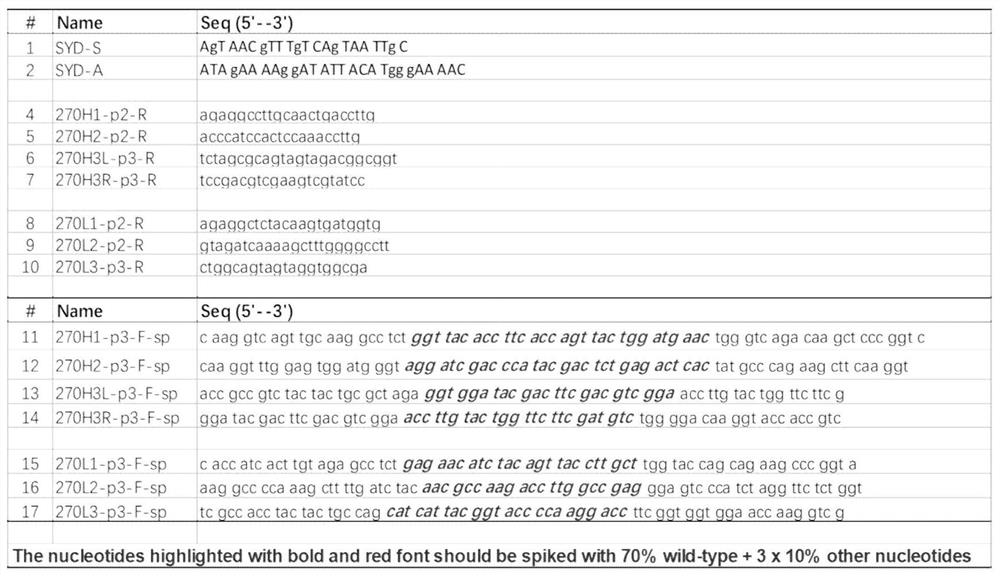
[0076] 1. Mutant antibody library design and construction
[0077]The humanized antibody hum270 was selected as a template for affinity maturation, and its heavy chain variable region and light chain variable region were connected by GS linker to construct a single-chain variable fragment (scFv). The CDR region of the single-chain antibody is the target of affinity maturation transformation, while the framework region will remain unchanged in the affinity maturation transformation. A total of 6 amino acids in the CDR region of the light and heavy chains (Table 1) were mutated, and mutation libraries were constructed respectively.
[0078...
Embodiment 3
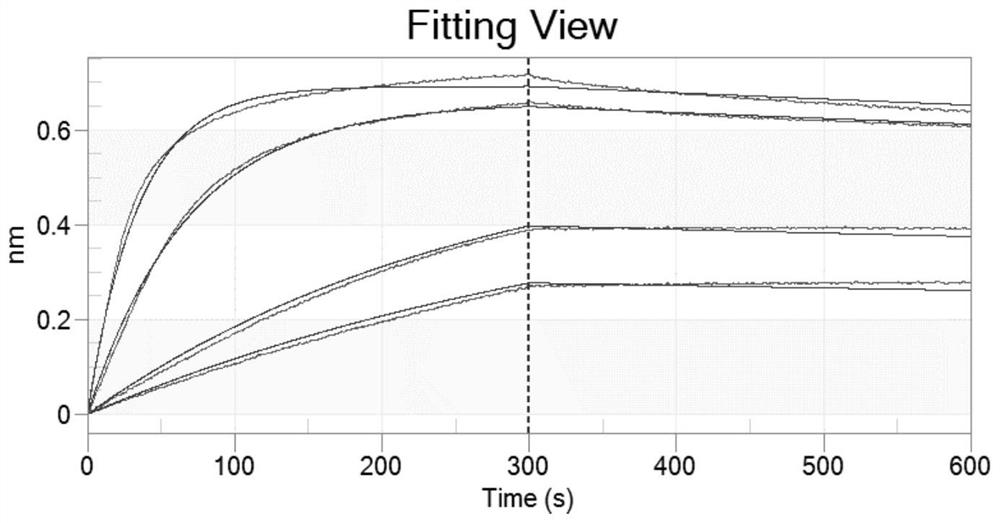
[0107] Example 3. Expression and affinity analysis of anti-NKG2a and PD-L1 bispecific antibody
[0108] 1. Bispecific antibody construction and affinity analysis
[0109] By PCR, the N-terminal of the nucleotide sequence encoding the anti-PD-L1 blocking humanized Nanobody F2 was connected to the C-terminal of the heavy chain nucleotide sequence of the hum270 antibody via a linker peptide to obtain a heavy chain containing hum270 antibody. The coding sequence hum270-H-F2 of chain-F2, the coding sequence of hum270 antibody heavy chain-F2 (hum270-H-F2) and the coding sequence of hum270 antibody light chain hum270-L were respectively cloned into eukaryotic transient In the expression vector, the obtained expression plasmid was transferred into Escherichia coli for amplification, and the hum270-H-F2 and hum270-L expression plasmids were isolated, and according to the operation instructions of the transfection reagent 293fectin (Cat: 12347019, Gibco), the plasmid Transformed into H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com