Therapeutic nanoconjugates and uses thereof
A nanoparticle and fusion protein technology, which is applied in the field of nanostructured protein materials, can solve the problems of complex and cumbersome antibody structures and obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0398] Synthesis of therapeutic nanoconjugates
[0399] Synthesis of T22-STM-H6-Aur therapeutic nanoconjugate.
[0400] T22-STM-H6 protein nanoparticles (in 166 mM NaCO3H pH=8 buffer) were mixed with maleimide-functionalized monomethyl auristatin E (MMAE) (resuspended in 50% PBS / 50% acetonitrile) at a protein:MMAE ratio of 1:50 for 4 h in a one-pot reaction. The resulting nanoconjugates were subsequently loaded with previously loaded Ni 2+ (NiCl 2 ) in a HiTrap chelating HP column (GE Healthcare), perform IMAC affinity chromatography, and wash with a washing buffer (20mM Tris, 500mM NaCl, 10mM imidazole pH=8) in the column for 30min in order to remove unreacted MMAE molecules, followed by one-step isocratic elution with elution buffer (20 mM Tris, 500 mM NaCl, 500 mM imidazole pH=8). The eluted nanoconjugates were then dialyzed (dyalized) against 166 mM NaCO3H pH=8 buffer using a 12-14 MWCO membrane O / N at 4°C to remove the elution buffer containing a high concentration ...
Embodiment 2
[0404] Characterization of the T22-STM-H6-Aur nanoconjugate and determination of the drug / nanoparticle ratio
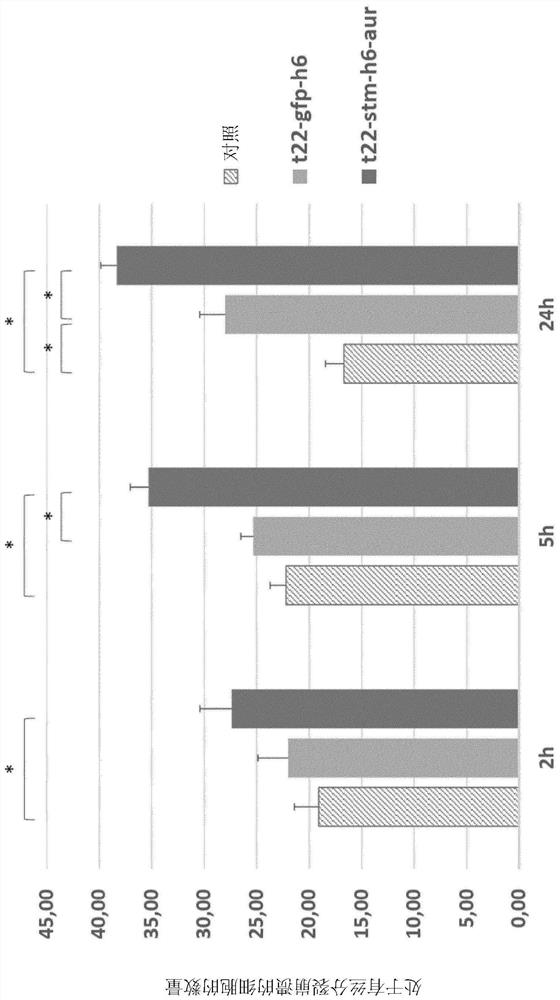
[0405] Nanoparticles of T22-GFP-H6 were formed from an average of 15 monomers, and each monomer of T22-GFP-H6-FdU bound about 4 molecules of oligo-FdU. This corresponds to about 60 molecules of oligo-FdU per nanoparticle. As for T22-GFP-H6-auristatin, the data show that it binds up to 30 auristatin per protein, which corresponds to about 450 auristatin per nanoparticle.
[0406] The nanoparticles of T22-STM-H6 are formed by an average of 15-20 monomers. Mass spectrometry indicates that no less than 3 auristatin molecules are bound to each protein monomer, and at least up to more than 15 auristatin molecules per monomer.
Embodiment 3
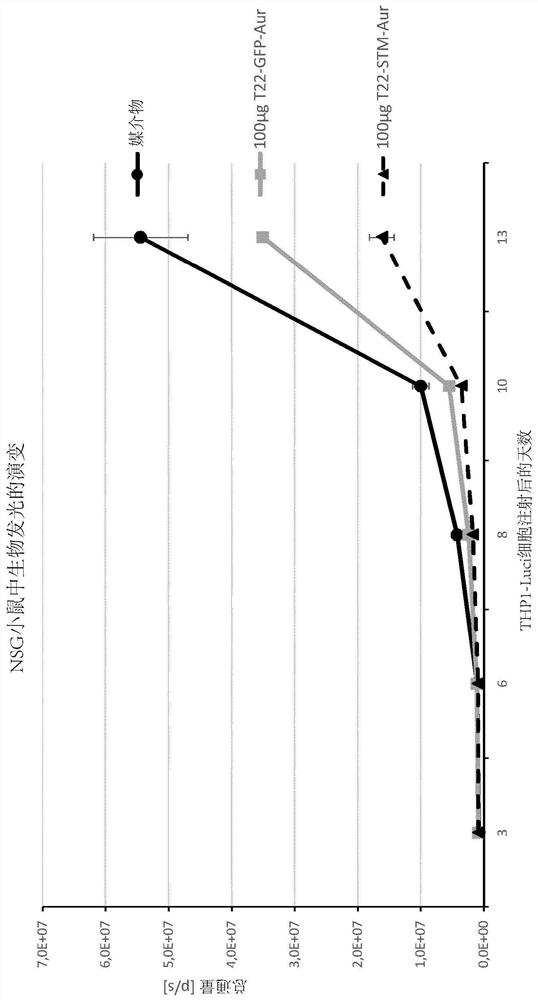
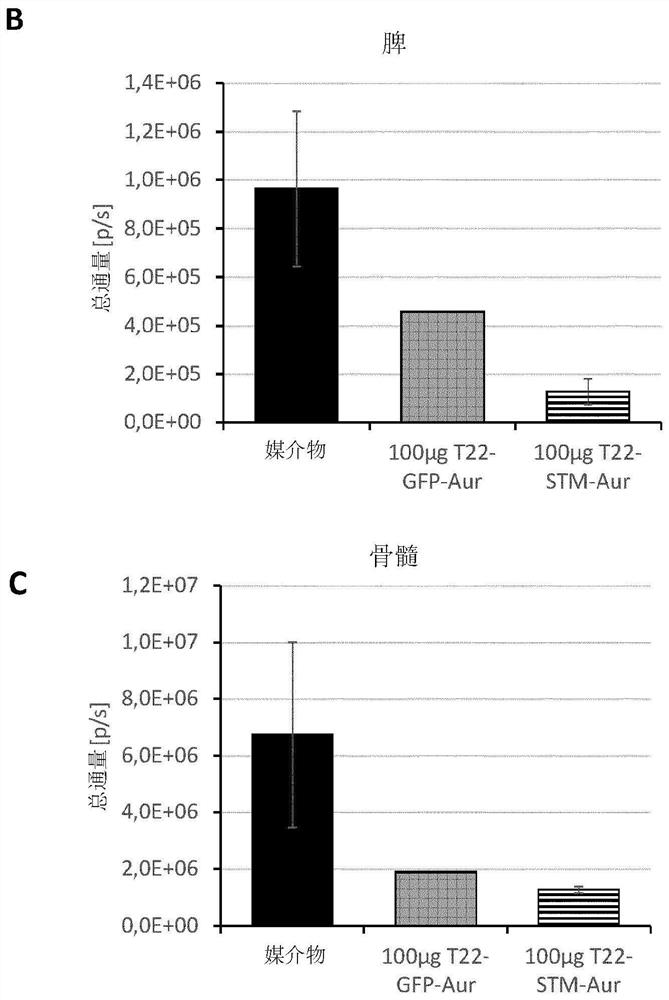
[0408] T22-STM-H6-Auristatin compared to T22-GFP-H6-Auristatin in a diffuse CXCR4+ AML mouse model Evaluation of antitumor effects of statins
[0409] method
[0410] THP1 cells transfected with luciferase (THP-1-Luci; 1 × 10 6cells / 200μL) intravenous (IV) injection of NSG (NOD-scid IL2Rγ 缺失(null) ) female mice (5 weeks old). Mice were randomly divided into three different experimental groups. One group (VEH; n=3) used the vehicle of the nanoconjugate (buffer NaCO 3 H+NaCl pH=8) IV injection, the experimental group (T22-STM-AUR; n=2) was injected IV with 100 μg of T22-STM-H6-aurestatin, and the positive control group (T22-GFP-AUR; n =1) Injected IV with 100 μg of T22-GFP-H6-aurestatin. All compounds were administered daily for a total of 9 doses. The evolution of AML spread in mice was monitored 3 times a week using the IVIS Spectrum device until the day of euthanasia. Animal body weights were measured on the same day as the BLI assay. Euthanize all mice on the d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com