Preparation method of heterogeneity 3D tumor composite multicellular sphere invasion model
A technology of tumor cells and multi-cells, which is applied in the field of preparation of heterogeneous 3D tumor compound multi-cell spheroid invasion models, can solve the problems of high cost of model preparation and difficult technical operation, and achieve low cost, good verification effect, and easy preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] According to a preferred embodiment of the present invention, SCC47 cells are used to construct a 3D tumor composite multicellular spheroid invasion model, as follows:
[0035] Cell source: Obtained from West China State Key Laboratory of Stomatology, Sichuan University.
[0036] Reagent consumables / instruments
[0037]
[0038]
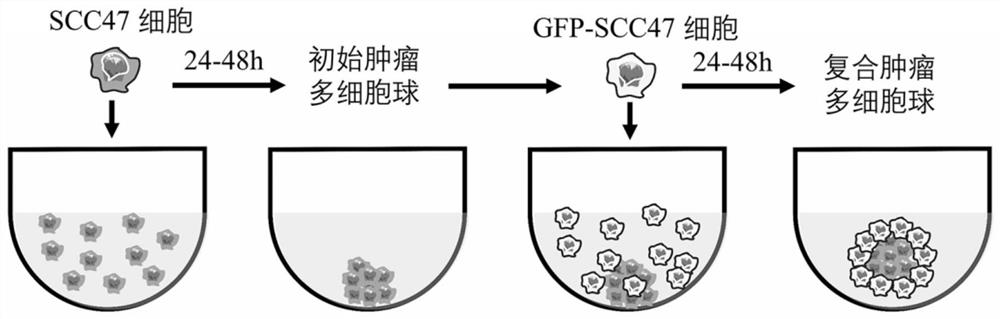
[0039] The model construction process is as follows, such as figure 1 Shown:
[0040] Step 1: Obtaining of single-cell suspension: the SCC47 tumor cells cultured to cover the bottom of the culture flask by 80%-90%, washed 3 times with PBS, added 1ml of 0.25% trypsin, and digested in a 37°C incubator ( About 2-5 minutes), add 4ml of complete medium, gently pipette to obtain a single cell suspension, centrifuge at 1000rpm for 5min, discard the supernatant, add complete medium to resuspend the cells.
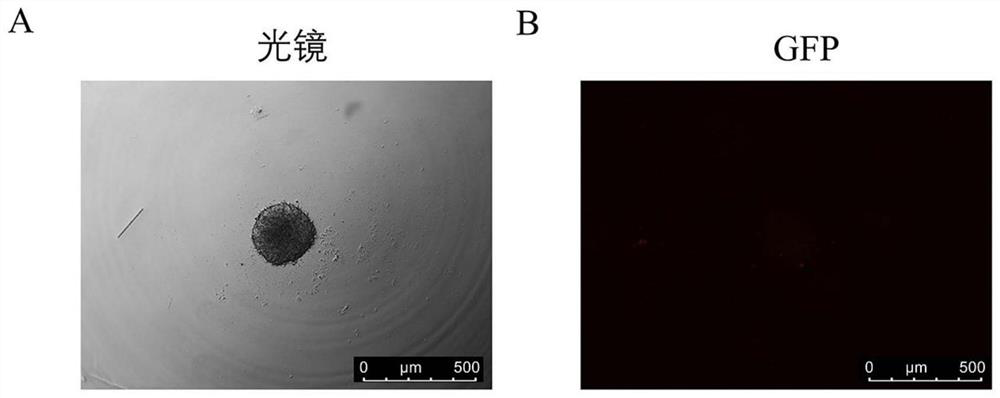
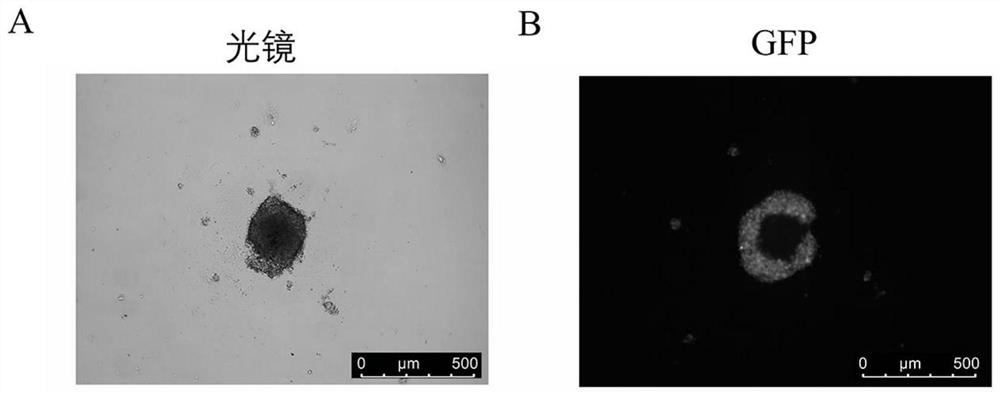
[0041] Step 2: Formation of initial multicellular spheres: Count the single-cell suspension obtained in the previous step using a countin...
Embodiment 2
[0050] The difference from Example 1 is that the cells in step 4 are SCC47 tumor cells transfected with a PD-L1 overexpression plasmid, and the transfection method is as follows:
[0051] PD-L1 overexpression plasmid transfection: SCC47 cells in the logarithmic growth phase were inoculated in 6-well plates so that the adherent cells reached 50-60% on the second day, and 2 μg of PD-L1 overexpression plasmid was pipetted into the 250μl Opti-MDM medium, pipette 8μl Lipofectamine 2000 reagent into 250μl Opti-MDM medium, incubate for 10 minutes, then gently mix the above liquid, incubate at room temperature for 20 minutes, add to the cells and supplement the medium, and incubate for 6 hours Afterwards, fresh complete medium was replaced, and culture was continued for 24 hours.
Embodiment 3
[0053] The difference from Example 1 is that the cells in Step 4 are SCC47 tumor cells transfected with a control plasmid, and the transfection method is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com