Bioconjugate vaccines' synthesis in prokaryotic cell lysates
A cell lysate, cell-free technology, used in vaccines, intact cells/viruses/DNA/RNA components, bacterial antigen components, etc., and can solve problems such as no proof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
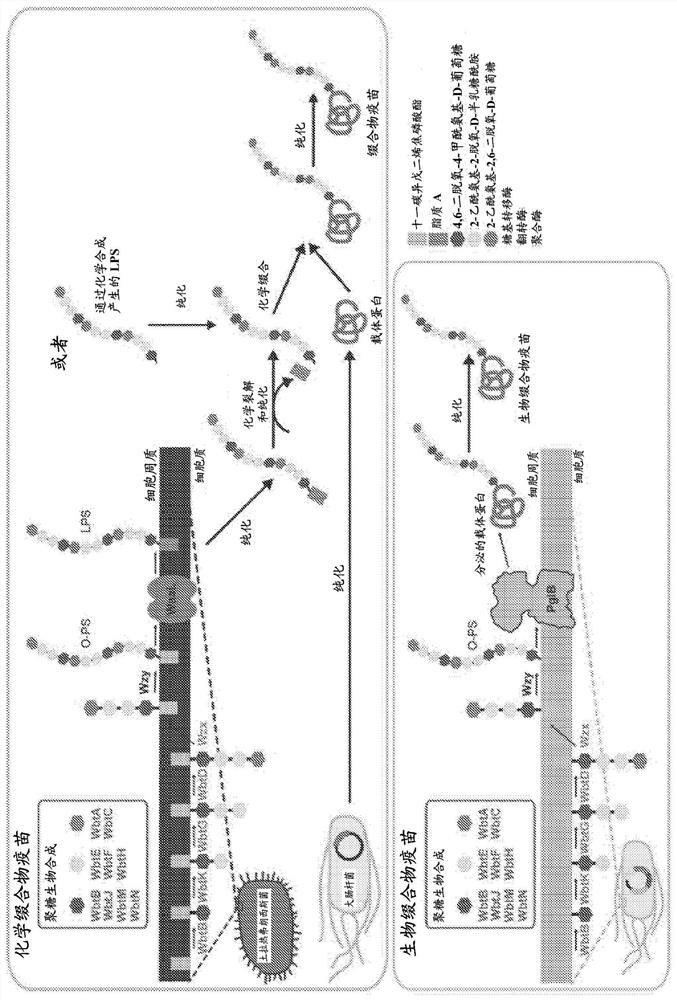
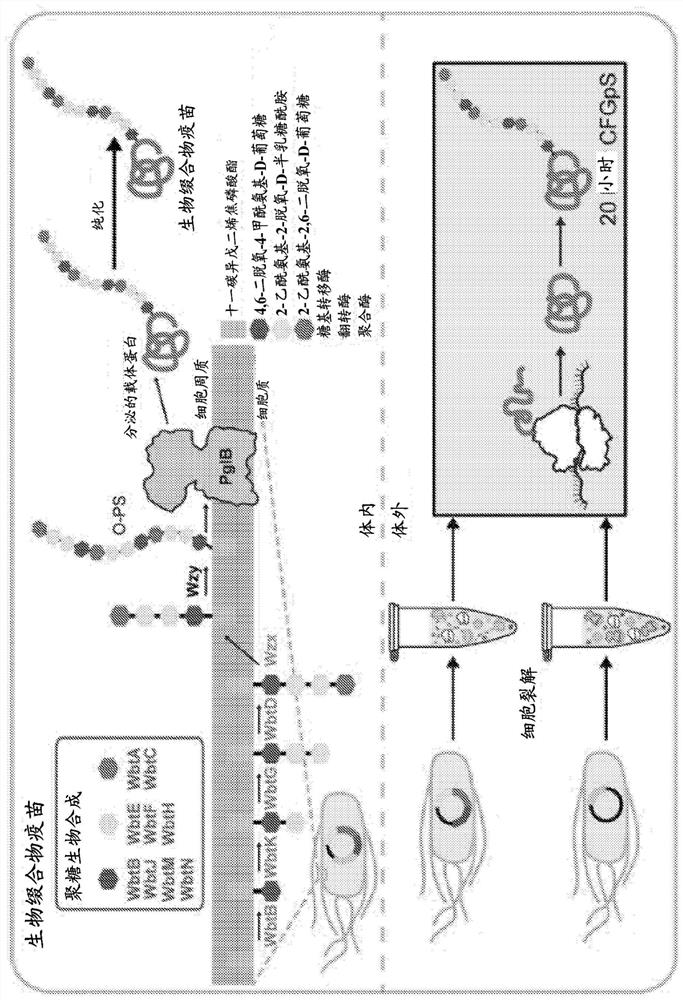
[0114] The in vitro bioconjugate vaccine preparation method disclosed in the present invention has demonstrated advantages in rapid, modular and portable vaccine formulation and preparation over existing methods. This system addresses the limitations of existing preparation methods, making it an attractive alternative or complementary strategy for antimicrobial vaccine preparation. Given the growing healthcare problem caused by antibiotic-resistant bacterial infections, this method has the potential to be very valuable for the development, preparation and distribution of new vaccines against a wide variety of pathogenic bacterial strains. Advantages of the methods of the present disclosure include: on-demand expression of bioconjugate vaccines; shaping of novel bioconjugate vaccine candidates; shaping of novel bioconjugate vaccine production pathways; distribution of bioconjugate vaccines to resource-poor settings.
[0115] In conclusion, we disclose the first prokaryotic cell...
Embodiment approach 1
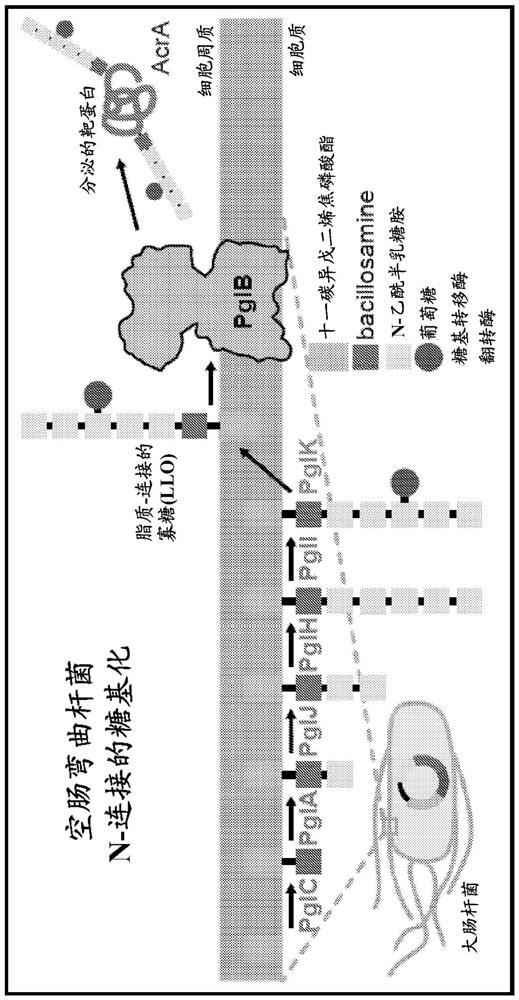
[0119] Embodiment 1. A method of synthesizing an N-glycosylated recombinant protein carrier that can optionally be used as a bioconjugate immunogen or a vaccine, the method comprising performing the synthesis of the recombinant protein carrier Coordinated transcription, translation and N-glycosylation, thereby providing said N-glycosylated recombinant protein carrier, said recombinant protein carrier can be used as a bioconjugate immunogen or vaccine, wherein said N-glycosylated recombinant The protein carrier comprises: (i) a consensus sequence (optionally inserted in said protein carrier) N-X-S / T, where X can be any natural or unnatural amino acid except proline; and (ii) derived from at least one At least one antigenic polysaccharide of bacteria N-linked to said recombinant protein carrier, wherein said at least one antigenic polysaccharide is optionally at least one bacterial O-antigen, optionally from Escherichia coli or Francisella tularensis and the bioconjugate vaccine...
Embodiment approach 2
[0120] Embodiment 2. The method of embodiment 1, wherein the carrier protein is an engineered variant of E. coli maltose binding protein (MBP).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com