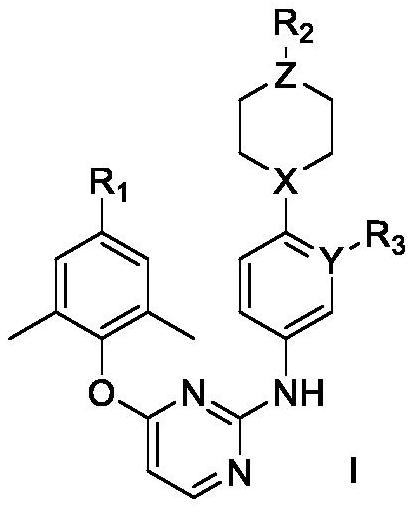
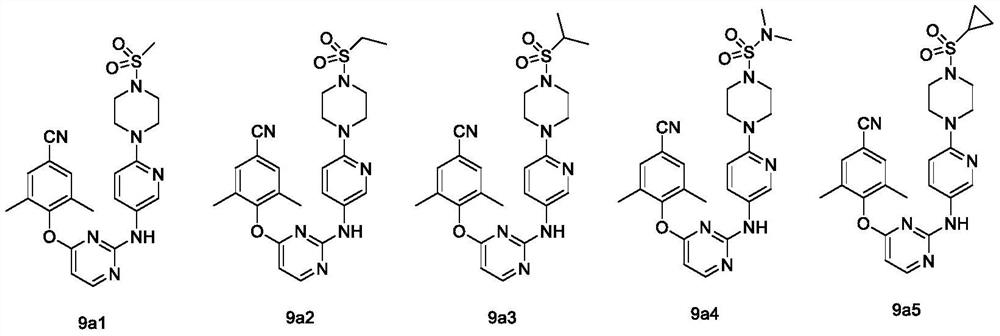
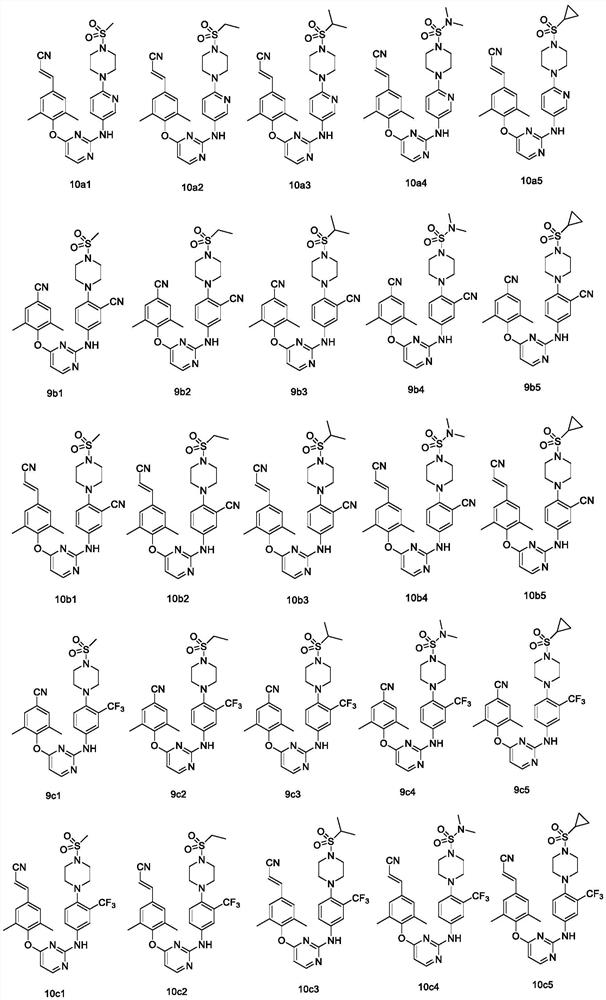
Diaryl pyrimidine HIV-1 reverse transcriptase inhibitor containing six-membered non-aromatic ring as well as preparation method and application of diaryl pyrimidine HIV-1 reverse transcriptase inhibitor
A technology of diarylpyrimidines and reverse transcriptase inhibition, applied in the field of medicine, can solve the problems of large oral dose, low oral bioavailability, toxic and side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (2)
[0041]
[0042] 2,4-Dichloropyrimidine 1 (1.49 g, 0.01 mol) and potassium carbonate (1.66 g, 0.012 mol) were dissolved in dimethylformamide (20 mL), and 4-hydroxy-3,5 - Dimethylbenzonitrile (1.47 g, 0.01 mol) and stirred at 50 °C for 4 h until the reaction was complete. Ice water (200 mL) was added and extracted with ethyl acetate (3 x 50 mL). The combined organic layers were washed with saturated brine, anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure and finally recrystallization using ethyl acetate and petroleum ether afforded the pure intermediate 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile 2. White solid, yield: 88.4%. 1 H NMR (400MHz, DMSO-d 6 )δ8.70(d, J=5.7Hz, 1H, pyrimidine-H), 7.75(s, 2H, Ph-H), 7.32(d, J=5.7Hz, 1H, pyrimidine-H), 2.10(s, 6H, Ph-CH 3 ×2). ESI-MS:m / z 259.97(M+H) + ,C 13 h 10 ClN 3 O(259.05).
Embodiment 2
[0043] Example 2: Preparation of 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzaldehyde (3)
[0044]
[0045] The preparation method is the same as in Example 1, except that the raw material 4-hydroxyl-3,5-dimethylbenzonitrile (1.47g, 0.01mol) is replaced by 4-hydroxyl-3,5-dimethylbenzaldehyde ( 1.50 g, 0.01 mol). White solid, yield: 81.4%. 1 HNMR (400MHz, DMSO-d 6 )δ9.97(s,1H,CHO),8.70(d,J=5.7Hz,1H,pyrimidine-H,Ph-H),7.77(s,2H),7.30(d,J=5.7Hz,1H, pyrimidine-H),2.15(s,6H,Ph-CH 3 ×2).ESI-MS:m / z263.10(M+H) + ,C 13 h 11 ClN 2 o 2 (262.69).
Embodiment 3
[0046] Example 3: Preparation of (E)-3-(4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylphenyl)acrylonitrile (4)
[0047]
[0048] Dissolve diethyl cyanomethylphosphate (0.092mL, 0.57mmol) in 5mL of tetrahydrofuran, add potassium tert-butoxide (0.0064g, 0.57mmol) under ice-cooling conditions, and activate for 30min, the 4-((2-chloro Pyrimidin-4-yl)oxy)-3,5-dimethylbenzaldehyde 3 (0.10 g, 0.38 mmol) in tetrahydrofuran (3 mL) was slowly added dropwise to the reaction liquid, and reacted at room temperature for 4 h. The solvent was evaporated under reduced pressure, the residue was added with 30 mL of water, extracted with ethyl acetate (3×10 mL), the organic phases were combined, washed with saturated sodium chloride solution (3×20 mL), dried over anhydrous sodium sulfate; filtered and concentrated under reduced pressure, Finally recrystallization using ethyl acetate and petroleum ether afforded pure intermediate (E)-3-(4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylphenyl)propene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com