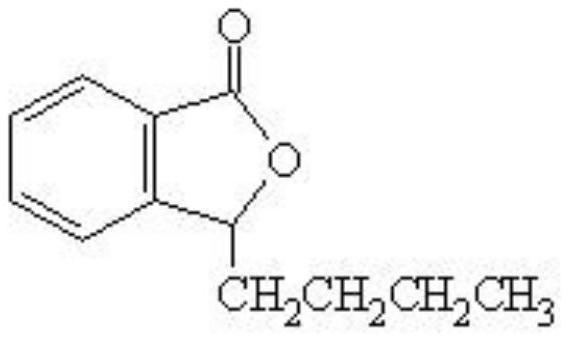
Stable butylphthalide small squirt, and preparation method and application thereof
A technology of butylphthalide and small water injection, which is applied in the field of preparation of small water injection of butylphthalide to treat cerebral apoplexy, can solve the problems of inconvenient clinical use and poor stability of butylphthalide injection, etc. Achieve the effects of easy use and portability, good safety, and scientific design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example discloses the preparation method of the small water injection of butylphthalide (5mL: 25mg, 5mg / ml) of the present invention. Its preparation prescription (prescription quantity 100) is:
[0037]
[0038] Preparation method: Take the prescribed amount of purified water, boil and cool to 80°C. Weigh 75 g of sodium sulfobutylbeta cyclodextrin (degree of substitution 6.2-6.9, average degree of substitution 6.4, molecular weight 2145.2 g / mol), add it to 400 mL of purified water at 80°C, and stir to dissolve completely. Weigh 2.5 g of butylphthalide, add it to the sodium sulfobutylbeta cyclodextrin solution, stir in an oil bath at 80°C for 120 min to dissolve completely. Allow to cool to room temperature, add 0.2M hydrochloric acid solution to adjust the pH value to 4.5, and add purified water to 500mL. Filter through a 0.45 μm filter membrane with a circulating water-type multi-purpose vacuum pump. Fill the prepared solution at 5mL / bottle. After filling,...
Embodiment 2
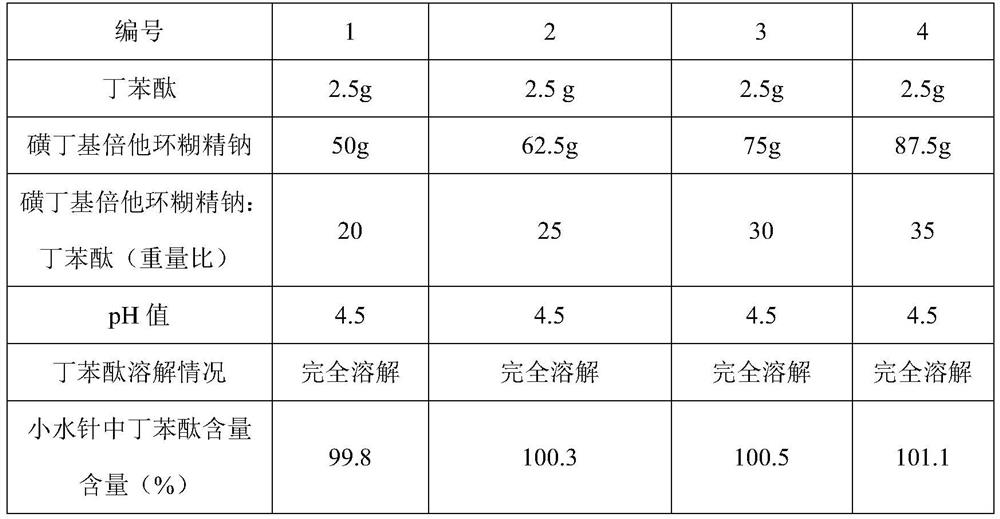
[0046] This embodiment discloses the investigation of the solubilizing ability of butylphthalide with the dosage of sulfobutylbeta cyclodextrin sodium, specifically:
[0047] According to the method of Example 1, small water injections of butylphthalide (5mL: 25mg, 5mg / ml) were prepared, the only difference being the amount of sodium sulfobutylbeta cyclodextrin. Then the properties of the prepared small water needles of butylphthalide and the content of butylphthalide in the small water needles were investigated.
[0048] The patent application number is CN2020113980997 "a stable butylphthalide sodium chloride injection, its preparation method and application", the molar ratio of butylphthalide to sulfobutylbeta cyclodextrin sodium is 1:1.60~2.20 , converted into a mass ratio of 1:18 to 25. The dosages of butylphthalide and sulfobutylbeta cyclodextrin sodium in Table 3 refer to the dosage settings.
[0049] Table 3 The comparison results of the solubilization ability of diff...
Embodiment 3
[0059] In this example, the effect of pH value on the stability of butylphthalide small water injection (5 mL: 25 mg, 5 mg / ml) at high temperature (60° C.) for 10 days was investigated.
[0060] According to the method of Example 1, the small water needles of butylphthalide are prepared, the difference is only that the adjusted pH values of the small water needles of butylphthalide are different, and the specific pH values of the prepared small water needles of butylphthalide are shown in Table 5. Table 5 shows the results of the impurity content in the small water needle.
[0061] The different pHs of table 5 affect the result table of embodiment prescription stability
[0062] Numbering 1 2 3 4 5 6 7 8 pH value 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 Maximum Simplex % 0.05 0.02 0.021 0.023 0.22 0.63 0.90 1.03 Total Miscellaneous % 0.10 0.02 0.023 0.025 0.29 0.69 0.95 1.05
[0063] It can be seen from Table 5 that ther...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com