Bilastine orally disintegrating tablet and preparation method thereof
A technology of disintegrating tablets and flakes, which is applied in the field of bilastine orally disintegrating tablets and its preparation, can solve the problems of large variation range between contents, black spots on the surface of tablets, and large variation range of contents, etc., to achieve potential Low risk, good durability, good taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
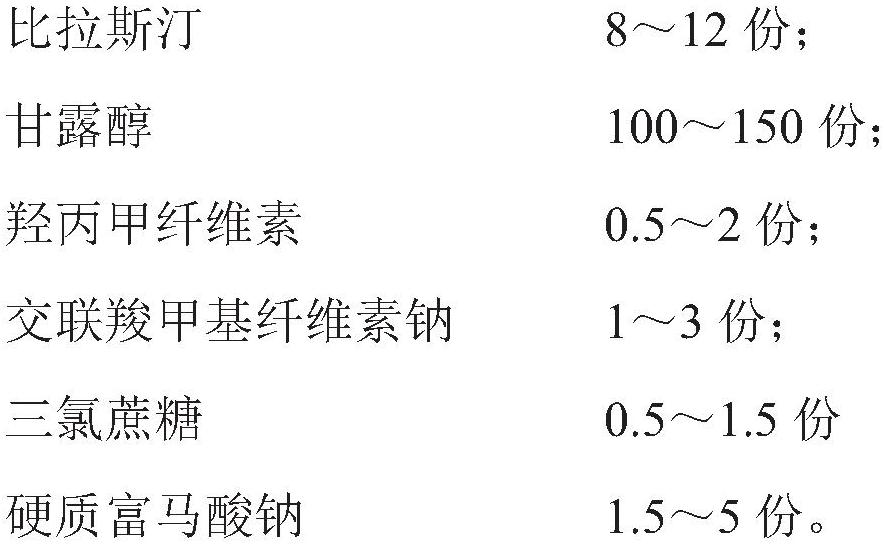
[0060] The prescription of embodiment 1-6 is as shown in table 3:
[0061] The prescription of table 3 embodiment 1-6
[0062]
[0063]
[0064] In Table 3, the mannitol used in Example 1 is the mannitol 160C produced by Roquette, France, and the mannitol used in Example 2 is the mannitol Granutol F produced by Japan Furende Sangyo Co., Ltd., and the mannitol used in Example 3. The mannitol is the mannitol 25C that French Roquette produces, and the mannitol that embodiment 4 uses is the mannitol 50C (the mannitol particle diameter of embodiment 1-4 is shown in Table 1) that French Roquette produces, and embodiment 5 The mannitol used is the mannitol produced by Roquette, France. After crushing at 160C, the particle size is controlled to 48.86μm50 90 50 90 <134.72 μm. The pulverized mannitol was confirmed to be in the form of flakes through a microscope.
[0065] The technological process of embodiment 1-6:
[0066] (1) Weigh the prescribed amount of hypromellose and a...
Embodiment 7
[0072] Embodiment 7 quality evaluation
[0073] 1. Detection method of content uniformity: take 10 tablets of the comparative example and the embodiment tablet respectively, put 10 tablets in 10 measuring bottles respectively, add solvent to dissolve and dilute the main ingredient to make about 1 ml of Bilas The need testing solution of bilastine 0.5mg is injected into the high performance liquid chromatograph, and the chromatogram is recorded; the reference substance solution containing about 0.5mg of bilastine is prepared in the same way, and each single dose is calculated by the peak area according to the external standard method The indicated amount is 100% relative content.
[0074] 2. Moisture absorption weight gain detection method: take 10 tablets of the comparative example and the embodiment, accurately weigh its weight m1, place it under the environment of RH90%±5% for 10 days, accurately weigh its weight m2, and calculate the weight gain percentage for
[0075] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com