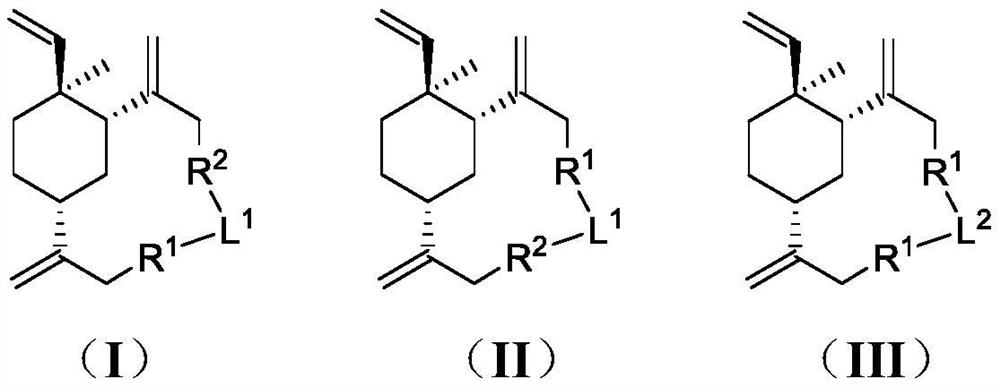
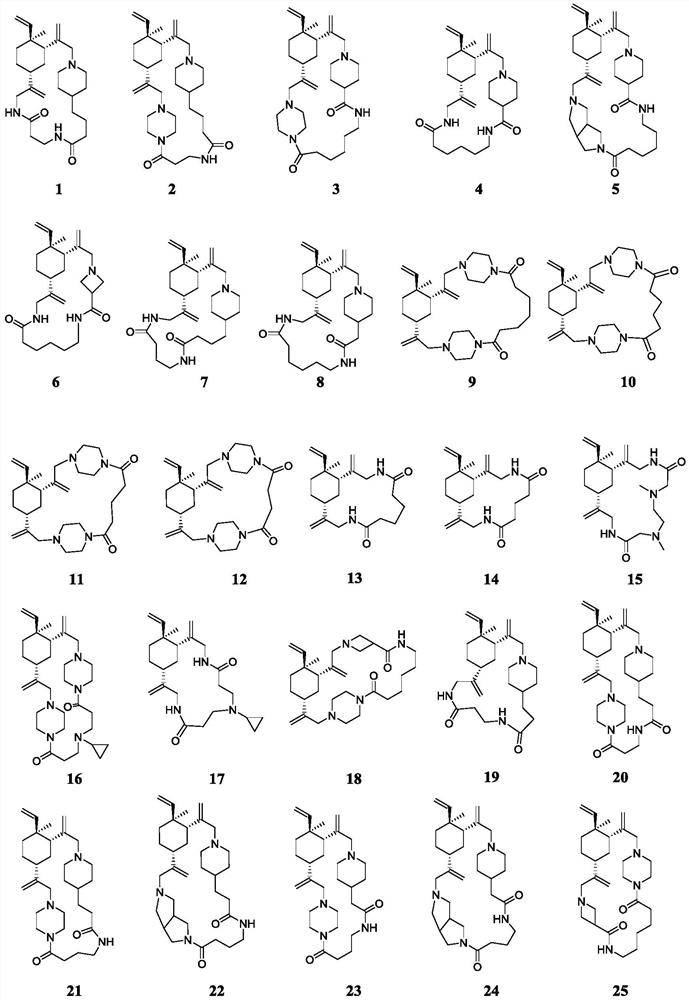
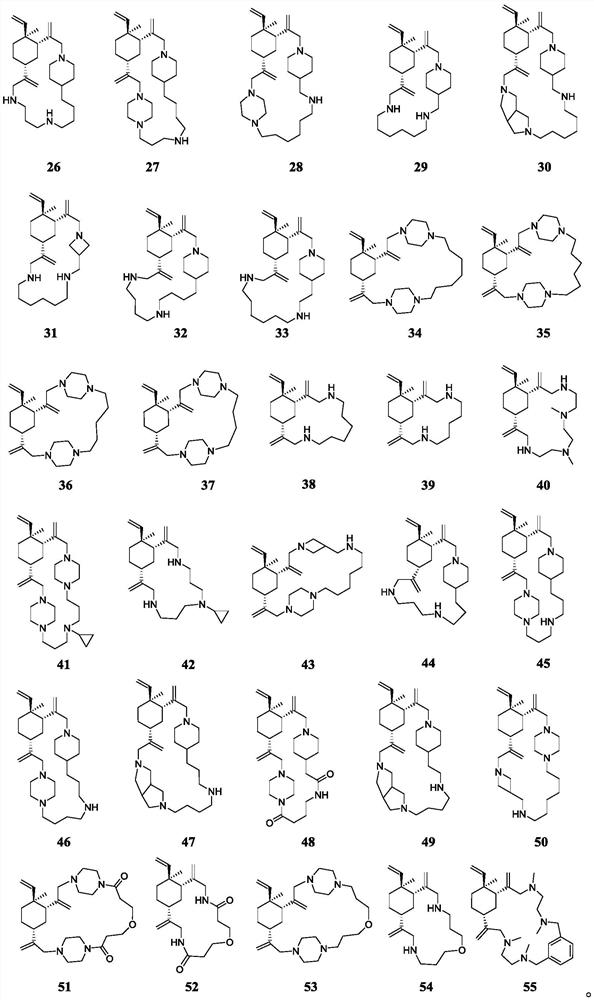
Beta-elemene macrocyclic derivative as well as preparation method and application thereof
A technology of macrocyclic derivatives and elemene, which is applied in the direction of drug combination, pharmaceutical formulation, bulk chemical production, etc., can solve the problems of poor water solubility, low polarity of elemene, and low bioavailability of oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of compound 1
[0084]
[0085] At room temperature, add 1b (536mg, 3.84mmol) to compound 1a (1042mg, 3.84mmol) in anhydrous N,N-dimethylformamide (DMF) (8mL) solution, after stirring and clarification, add N,N-diisopropylethylamine (DIPEA) (1092mg, 8.45mmol), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluoro Phosphate (HATU) (1460 mg, 3.84 mmol), stirred overnight. DMF was distilled off under reduced pressure, ice water (40 mL) was added to quench the reaction, and extracted with ethyl acetate (15 mL x 3). The combined organic phases were washed successively with water (10 mL x 2) and saturated brine (10 mL x 2), and then dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the filtrate was concentrated under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (eluted with ethyl acetate / petroleum ether / triethylamine (0.1%) system) to obtain yellow oil...
Embodiment 2
[0091] Embodiment 2: the preparation of compound 2
[0092]
[0093] Referring to the synthesis method of Example 1, compound 2f (252 mg, yield 46%) was obtained. 1 H NMR (500MHz, CDCl 3 )δ6.13(t,J=4.9Hz,1H),5.79(dd,J=17.5,10.8Hz,1H),5.00(s,1H),4.95–4.81(m,4H),4.74(s,1H ),3.68(s,3H),3.50(q,J=6.1Hz,2H),3.39(s,4H),2.97(d,J=13.9Hz,1H),2.90(q,J=13.3Hz,2H ),2.81–2.71(m,2H),2.59(d,J=13.9Hz,1H),2.53(t,J=6.0Hz,2H),2.31(s,4H),2.20(dd,J=12.7, 3.1Hz, 1H), 2.14–2.09(m, 2H), 2.07(d, J=12.1Hz, 1H), 1.89(t, J=11.3Hz, 1H), 1.56(dq, J=42.1, 13.9, 13.0 Hz,9H),1.44(s,9H),1.40(d,J=12.7Hz,1H),1.30–1.05(m,6H),0.98(s,3H).LCMS m / z643.0[M+H ] + .
[0094] Referring to the synthesis method of Example 1, compound 2f (245 mg, 0.38 mmol) was subjected to Boc deprotection, ester hydrolysis, and amide condensation in sequence to finally obtain white solid compound 2 (97 mg, yield 76%). 1 H NMR (400MHz, CDCl 3 )δ6.30–6.20(m,1H),5.76(dd,J=17.5,10.8Hz,1H),4.93–4.87(m,3H),4.86(d,J=1.2Hz,1H),4.84–4.82...
Embodiment 3
[0095] Embodiment 3: the preparation of compound 3
[0096]
[0097] Referring to the synthesis method of Example 1, compound 3c (1602 mg, yield 85%) was obtained in the first step. 1 H NMR (500MHz, CDCl 3 )δ5.59(s,1H),3.65(s,3H),3.24(q,J=7.0Hz,2H),2.72(t,J=11.0Hz,2H),2.30(t,J=7.4Hz, 2H), 2.19(tt, J=11.6, 3.7Hz, 1H), 1.78(d, J=12.6Hz, 2H), 1.66–1.58(m, 4H), 1.50(dt, J=14.8, 7.4Hz, 3H ), 1.44(s,9H), 1.39–1.28(m,3H).
[0098] The second step produced the hydrochloride (763mg, 2.61mmol) of compound 3d, LCMS m / z 257.2[M+H] + . The crude product was directly used in the next reaction without purification.
[0099] The third step yielded compound 3f (142 mg, yield 66%). 1 H NMR (500MHz, CDCl 3 )δ5.80(dd, J=17.5,10.8Hz,1H),5.58(t,J=5.1Hz,1H),5.01(s,1H),4.92(d,J=7.3Hz,2H),4.90– 4.81(m,2H),4.75(s,1H),3.65(s,3H),3.40(t,J=5.1Hz,4H),3.24(q,J=6.7Hz,2H),3.02(d,J =13.8Hz,1H),2.96–2.87(m,2H),2.87–2.78(m,2H),2.61(d,J=13.9Hz,1H),2.38–2.25(m,6H),2.21(dd, J=12.9, 3.2Hz, 1H), 2.11–1.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com