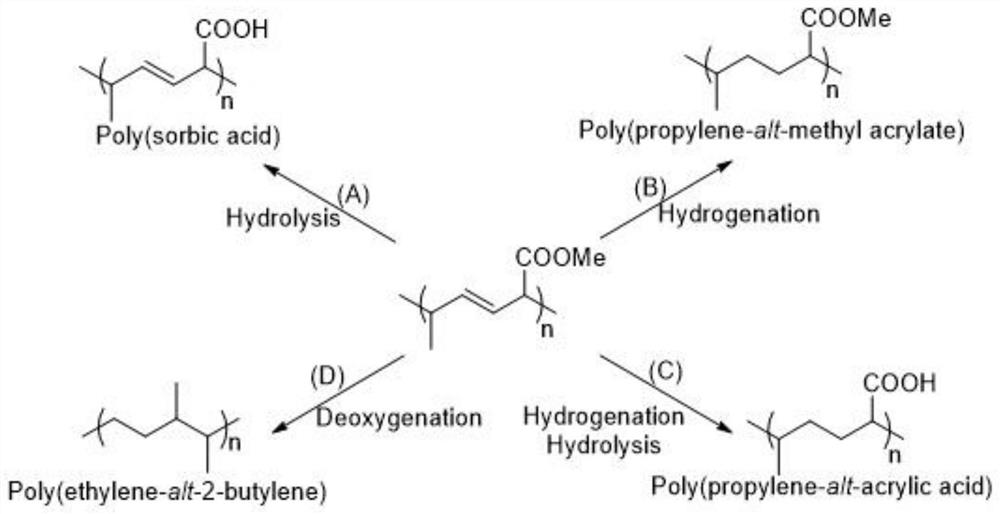
Application of Lewis base, sorbate polymer and derivative of sorbate polymer
A technology of Lewis base and sorbate, applied in the direction of adhesives, etc., can solve the problems of low initiation efficiency, low nucleophilicity, and inability to initiate polymerization reactions well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The invention provides a kind of preparation method of sorbic acid ester polymer, comprises the following steps:
[0062] Mix (E,E)-sorbate, Lewis acid, Lewis base and an organic solvent to perform 1,4-selective addition polymerization to obtain a sorbate polymer; the 1,4-selective addition The temperature of the polymerization reaction is -50~100℃;
[0063] The Lewis base is the Lewis base described in the above technical scheme.
[0064] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
[0065] In the present invention, the (E,E)-sorbate monomer preferably has the structure shown in formula III:
[0066]
[0067] In the formula III, R preferably includes methyl, ethyl, isopropyl, n-butyl, isobutyl, tert-butyl, phenyl, benzyl, trimethylsilyl, triethylsilyl, trimethylsilyl Oxysilyl group, triphenylmethyl group or methyl ethyl ether group; the (E,E)-sorbate ...
Embodiment 1
[0096] Dissolve 2.0 mmol of methyl sorbate (MS) in 500 μL of toluene, add 0.5 mL of LA toluene solution with a concentration of 0.08 mol / L (the amount of LA is 0.04 mmol) and stir for 1 min, add 500 mL of a solution with a concentration of 0.04 mol / L Toluene solution of NHO1 (the amount of LB is 0.02mmol), add toluene until the total volume of toluene is 2.0mL, 1,4-selective addition polymerization reaction at 25°C for 1h, to obtain sorbate polymer (PMS );
[0097] Among them, LA is (BHT) 2 AlPh; LB is NHO1; LB:LA:MS molar ratio=1:2:100;
Embodiment 2~15
[0099] The sorbate polymer was prepared according to the method of Example 1. The preparation conditions of Examples 2-15 were shown in Table 1, and other conditions not listed in Table 1 were the same as in Example 1.
[0100] The preparation conditions of table 1 embodiment 1~15
[0101]
[0102]
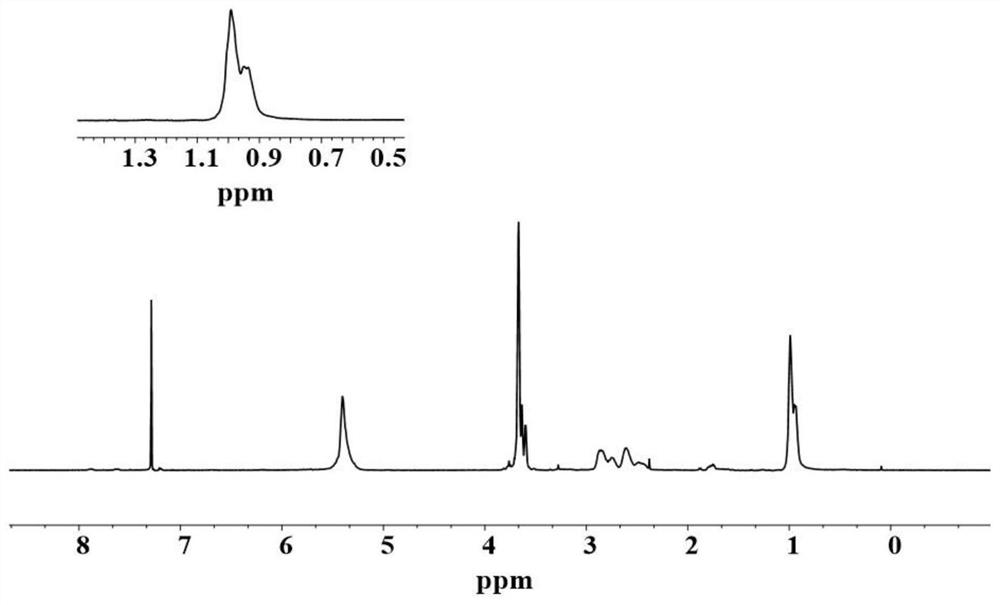
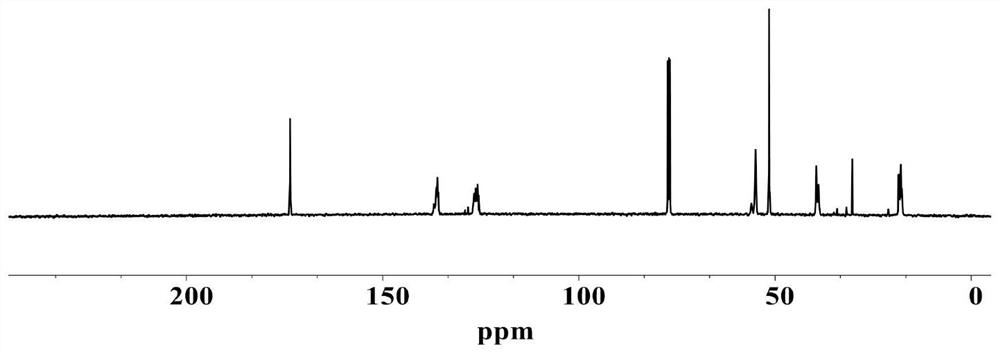
[0103] (1) Structural characterization of sorbate polymer
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com