Deuterated forms and derivatives of volinanserin
A technology of compounds and structural formulas, applied in the field of deuterated forms and derivatives of fliserin, which can solve problems such as reduced metabolic clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0085]
[0086]
[0087] In some embodiments, the compound is selected from any of the compounds described in Table 1 (above), wherein any atom not designated as deuterium is present in its natural isotopic abundance.
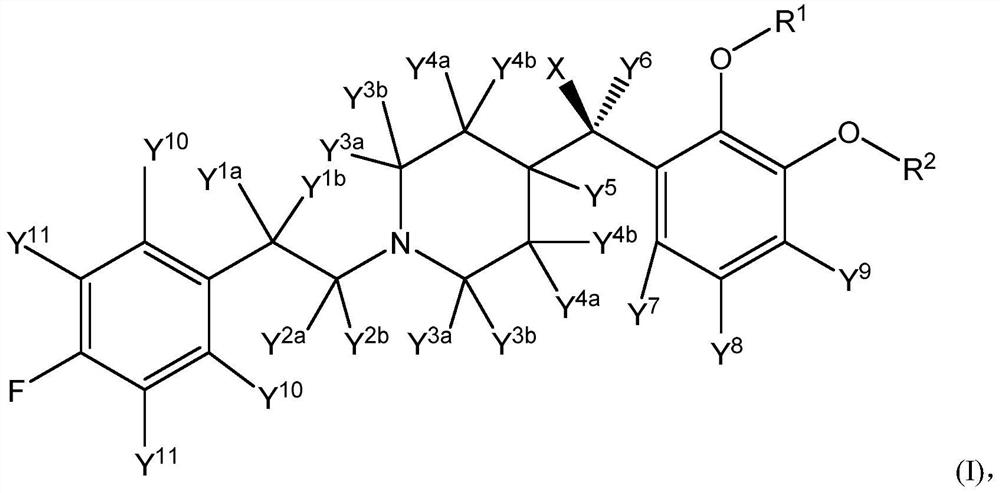
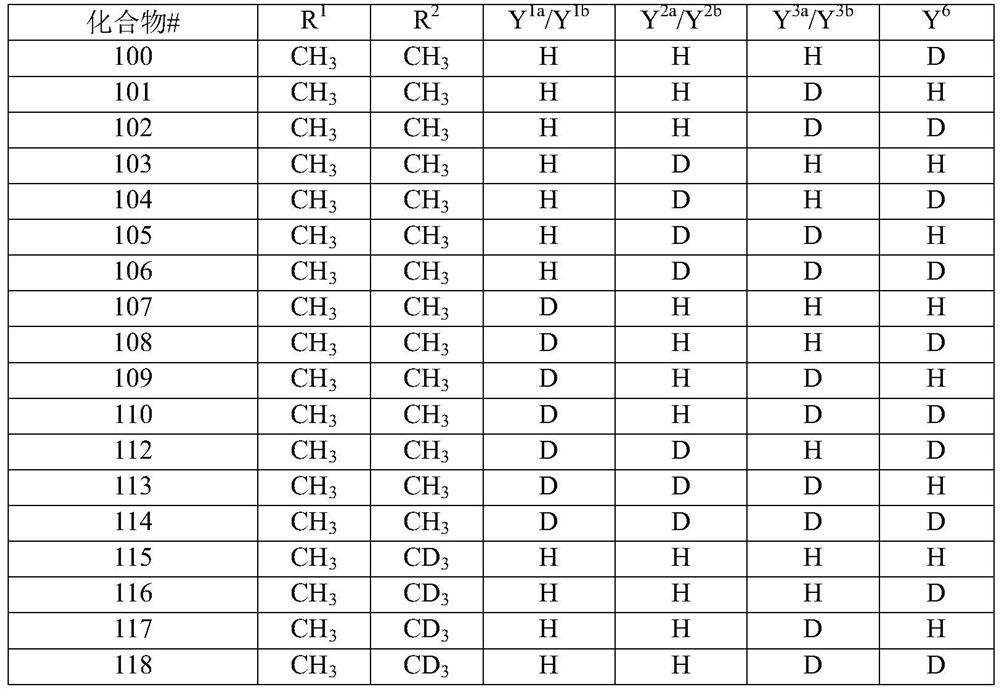
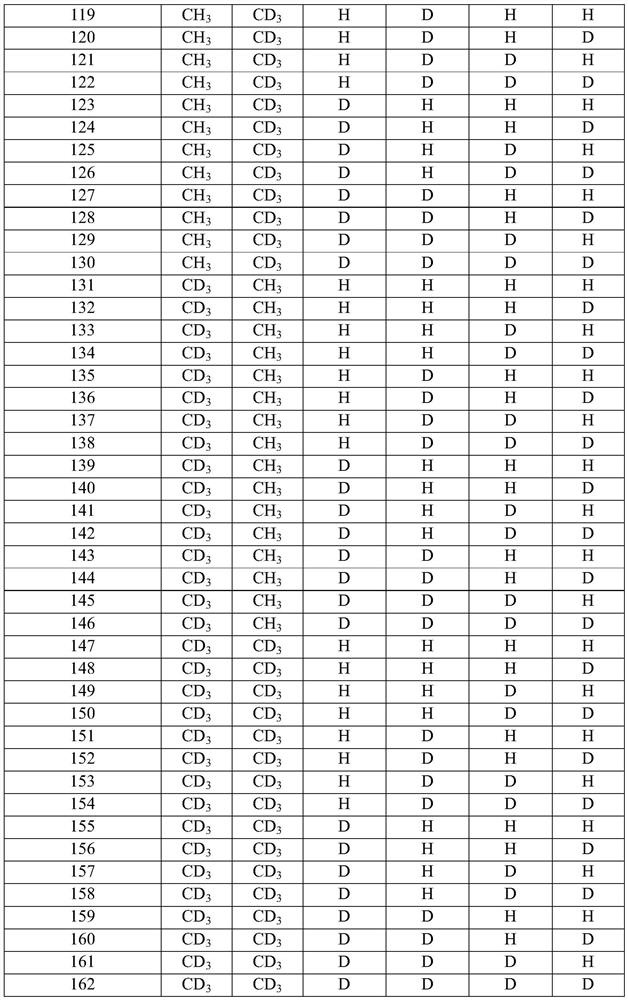
[0088] In some embodiments, the compound of structural formula (I) or structural formula (II) is selected from any of the compounds described in Table 2 (below), wherein X, when present, is -OH; Y 1a and Y 1b the same; Y 2a and Y 2b the same; Y 3a and Y 3b the same; and Y 4a , Y 4b , Y 5 , Y 7 , Y 8 , Y 9 , Y 10 and Y 11 each for hydrogen:
[0089] Table 2: Exemplary embodiments of structural formula (I)
[0090]
[0091]
[0092] In some embodiments, the compound is selected from any of the compounds described in Table 2 (above), wherein any atom not designated as deuterium is present in its natural isotopic abundance.
[0093] In some embodiments, the compound of structural formula (I) or structural formula (II) is selected from any ...
Embodiment 1
[0222] Example 1. Synthesis of (R)-(2,3-bis(methoxy-d 3 )phenyl)(1-(4-fluorophenethyl)piperidin-4-yl)methanol (Compound 147).
[0223] Scheme 6. Preparation of (R)-(2,3-bis(methoxy-d 3 )phenyl)(1-(4-fluorophenethyl)piperidin-4-yl)methanol (Compound 147).
[0224]
[0225] Step 1. 1,2-bis(methoxy-d 3 ) benzene (21b). To a solution of 1,2-dihydroxybenzene (20a) (30 g, 272.5 mmol) in anhydrous DMSO (250 mL) was added KOH (61.2 g, 1090 mmol) at room temperature, followed by methyl iodide-d 3 (42.4 mL, 681.1 mmol, Sigma Aldrich, >99.5% atomic D). The reaction mixture was stirred overnight at room temperature. The reaction mixture was diluted with water (800 mL), and washed with CH 2 Cl 2 (4 x 600 mL) extraction. The combined organic layers were washed with water (3×1 L), dried (Na 2 SO 4 ), filtered and concentrated under reduced pressure. The residue was dried (vacuum oven) to afford 21b (36.4 g, 92%) as a yellow oil.
[0226] Step 2. 4-(2,3-bis(methoxy-d 3 ) Benzo...
Embodiment 2
[0235] Example 2. Synthesis of (R)-(1-(4-fluorophenethyl)piperidin-4-yl)(2-methoxy-3-(methoxy-d 3 ) phenyl) methanol (compound 115).
[0236] Scheme 7. Preparation of (R)-(1-(4-fluorophenethyl)piperidin-4-yl)(2-methoxy-3-(methoxy-d 3 ) phenyl) methanol (compound 115).
[0237]
[0238] Step 1. 2-((1-(4-fluorophenethyl)piperidin-4-yl)(hydroxy)methyl)-6-(methoxy-d 3 ) phenol (30b). To a solution of 6b (2.5 g, 7 mmol) in anhydrous THF (100 mL) was added a 1.0 M solution of lithium tri-sec-butylborohydride in THF (27 mL, 27 mmol) at 0°C. The reaction mixture was stirred at 0°C for 2 hours, then heated at 70°C overnight. The reaction mixture was cooled to 0 °C and quenched with water (150 mL). Separate the layers with Et 2 The aqueous layer was extracted with O (2 x 150 mL). The combined organic layers were washed with saturated saline solution and dried (Na 2 SO 4 ), filtered and concentrated under reduced pressure. Chromatography (Interchim automated chromatography s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com