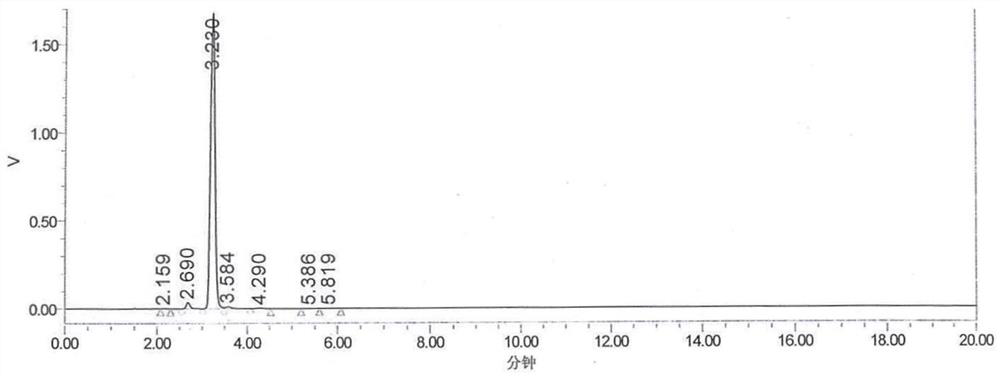
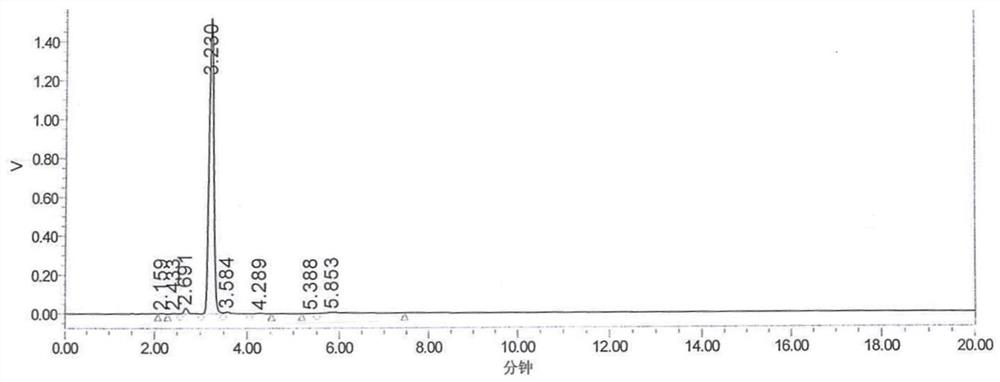
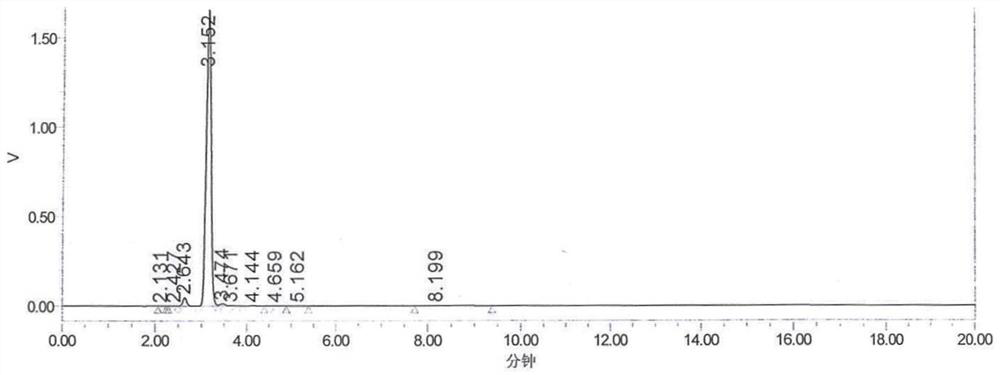
Synthesis process of OAB-14
A technology of OAB-14, 1.OAB-14, applied in the preparation of organic compounds, carbamic acid derivatives, urea derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]Add OAB-14-7 (21.44g), 200ml dichloromethane, anhydrous potassium carbonate (17.33g, 2.5eq.) into a 500ml four-neck flask, stir well, add phenyl chloroformate (20.1g, 2.5eq.), after dripping, the temperature was raised to reflux, and the temperature was kept at reflux for 5.5h.
[0077] Add 200ml of purified water, and cool to room temperature in an ice bath. Suction filtration, 200ml of purified water to wash the filter cake, and then 200ml of dichloromethane to wash the filter cake. The filter cake was beaten again with 200ml of dichloromethane for 1h. Suction filtration and drying yielded 21.47 g of white solid.
[0078] The HPLC detection content was 97.197%, and OAB-14-7 remained 1.731%, and the yield was 81.17%.
[0079] Add triethylamine (7.6g, 3eq.), ethylenediamine (9g, 6eq.) and 100ml tetrahydrofuran to a 250ml four-necked flask, stir evenly, and then add the phenyl ester prepared in the previous step in batches at room temperature solid (10 g), after the a...
Embodiment 2
[0082] Add OAB-14-7 (21.44g), 200ml dichloromethane, anhydrous potassium carbonate (20.80g, 3.0eq.) into a 500ml four-necked flask, stir evenly, add phenyl chloroformate (24.2g, 3.0eq.), after dripping, the temperature was raised to reflux, and the temperature was kept at reflux for 5h.
[0083] Add 200ml of purified water, and cool to room temperature in an ice bath. Suction filtration, 200ml of purified water to wash the filter cake, and then 200ml of dichloromethane to wash the filter cake. The filter cake was beaten again with 200ml of dichloromethane for 1h. Suction filtration and drying yielded 18.97 g of white solid.
[0084] The HPLC detection content was 95.83%, and OAB-14-7 remained 1.519%, and the yield was 70.71%.
[0085] Add triethylamine (5.07g, 2eq.), ethylenediamine (12g, 8eq.) and 100ml tetrahydrofuran into a 250ml four-necked flask, stir well, and then add the phenyl ester prepared in the previous step in batches at room temperature solid (10 g), after t...
Embodiment 3
[0088] Add OAB-14-7 (21.44g), 200ml dichloromethane, anhydrous potassium carbonate (24.26g, 3.5eq.) into a 500ml four-necked flask, stir well, add phenyl chloroformate (28.2g, 3.5eq.), after dripping, the temperature was raised to reflux, and the temperature was kept at reflux for 4.5h.
[0089] Add 200ml of purified water, and cool to room temperature in an ice bath. Suction filtration, 200ml of purified water to wash the filter cake, and then 200ml of dichloromethane to wash the filter cake. The filter cake was beaten again with 200ml of dichloromethane for 1h. Suction filtration and drying yielded 21.63 g of white solid.
[0090] The HPLC detection content was 96.466%, and OAB-14-7 remained 2.273%, and the yield was 81.16%.
[0091] Add triethylamine (8.87g, 3.5eq.), ethylenediamine (15g, 10eq.) and 100ml tetrahydrofuran into a 250ml four-necked flask respectively, stir evenly, and then add the phenyl ester prepared in the previous step in batches at room temperature Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com