Anti-PD-L1/VEGF fusion protein
A PD-L1 and fusion protein technology, applied in the field of fusion proteins, can solve the problems of slow tumor growth and inability to achieve metastasis, and achieve the effect of excellent blocking ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. Construction of anti-PD-L1 / VEGF bifunctional fusion protein
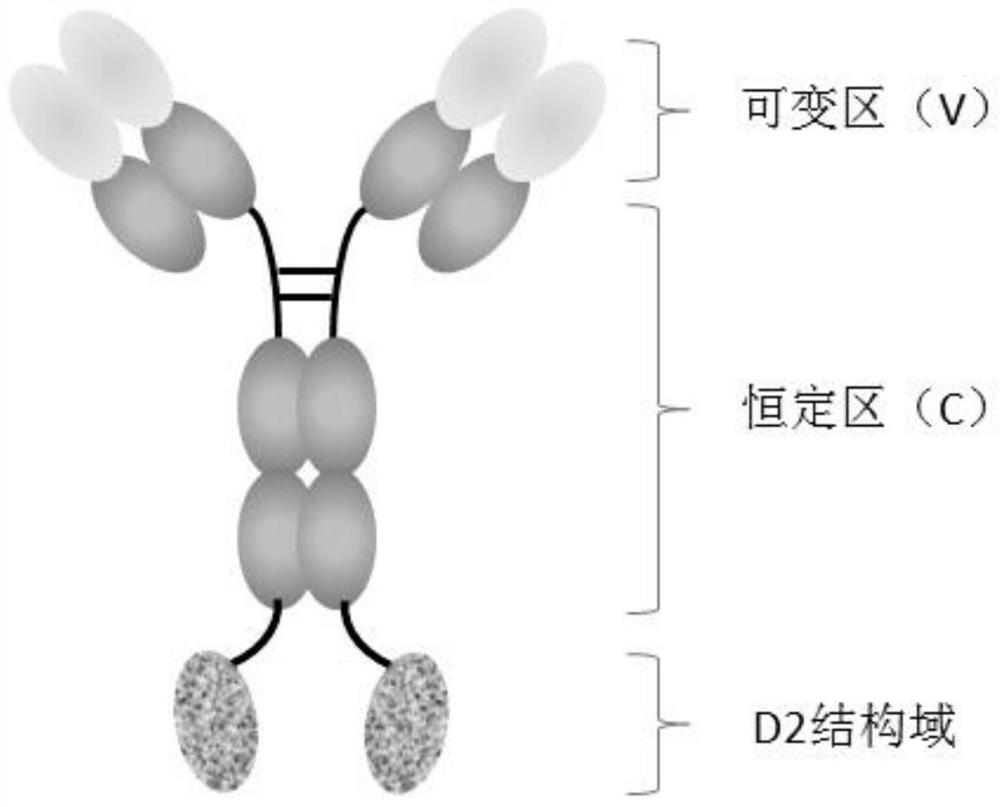
[0057] In the present invention, the anti-PD-L1 monoclonal antibody is connected in series with the D2 domain of VEGFR1 to construct an anti-PD-L1 / VEGF bifunctional fusion protein, and the structural diagram is as follows figure 1 shown.
[0058] fusion protein M8-D2
[0059] The N-terminus of the D2 domain of VEGFR1 (SEQ ID NO: 1) and the C-terminus of the heavy chain (SEQ ID NO: 2) of the anti-PD-L1 monoclonal antibody M8 are linked by a peptide linker L (SEQ ID NO: 3) , to obtain the heavy chain of the fusion protein (SEQ ID NO: 4), and the sequence of the light chain of the fusion protein is SEQ ID NO: 5.
[0060] Fusion protein M8-D2-M2
[0061] The N-terminus of the D2 domain of VEGFR1 (SEQ ID NO: 6) and the C-terminus of the heavy chain (SEQ ID NO: 2) of the anti-PD-L1 monoclonal antibody M8 are connected by a peptide linker L (SEQ ID NO: 3) , to obtain the heavy chain of the fusion prot...
Embodiment 2
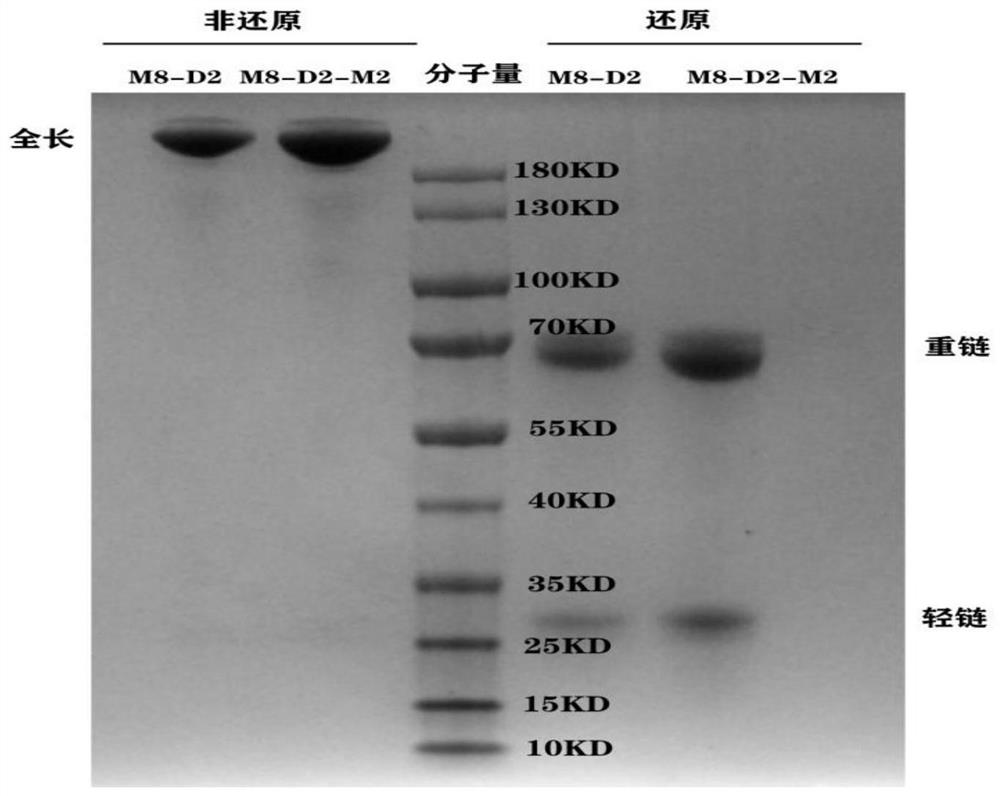
[0063] Example 2. Expression and purification of anti-PD-L1 / VEGF bifunctional fusion protein
[0064] The nucleic acid sequence of the heavy chain of M8-D2 is SEQ ID NO: 8, and the nucleic acid sequence of the light chain is SEQ ID NO: 9. The nucleic acid sequence of the heavy chain of M8-D2-M2 is SEQ ID NO: 10, and the nucleic acid sequence of the light chain is SEQ ID NO: 9. The DNA fragments of the heavy and light chains of the anti-PD-L1 / VEGF bifunctional fusion protein were subcloned into pcDNA3.4 vectors (purchased from thermofisher, A14697), and the recombinant plasmids were extracted and co-transfected into CHO cells and / or 293F cells. After the cells were cultured for 7 days, the culture solution was filtered by high-speed centrifugation and vacuum filtration with a microporous membrane, and then loaded onto the HiTrap MabSelect SuRe column, and the protein was eluted with 100mM citric acid, pH3.5 eluent in one step, and the target sample was recovered and dialyzed C...
Embodiment 3
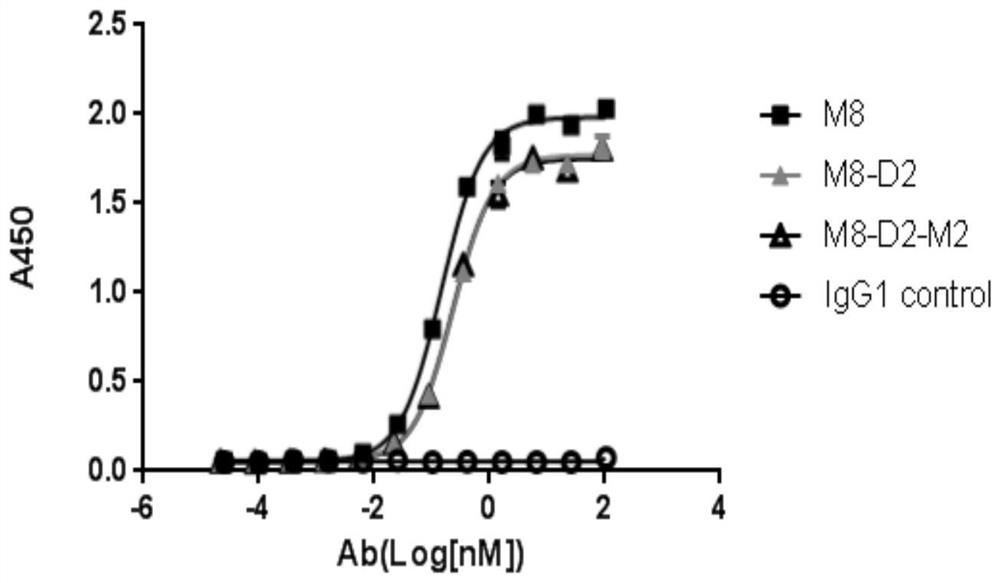
[0065] Example 3. Determination of the affinity of the anti-PD-L1 / VEGF bifunctional fusion protein to the antigen by enzyme-linked immunosorbent assay (ELISA) 3.1 ELISA detection of the affinity of the anti-PD-L1 / VEGF bifunctional fusion protein to PD-L1
[0066]Self-made recombinant PD-L1-ECD-Fc protein (refer to WO2018 / 137576A1 for the preparation method), coated with 100ng / well, overnight at 4°C. Wash the plate 3 times with PBST, add 200 μl / well blocking solution, place at 37°C for 1 hour, wash the plate once with PBST and set aside. Dilute the antibody with diluent to 100 μg / ml, and dilute it 4 times to form 12 concentration gradients (the highest concentration is 100000 ng / ml, the lowest concentration is 0.02 ng / ml), and add to the blocked ELISA plate in turn, 100 μl / well, 37 °C for 1 hour. Wash the plate three times with PBST, add HRP-labeled goat anti-human Fab antibody (purchased from abcam, Cat. #ab87422), and place at 37°C for 30 minutes. After washing the plate wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com