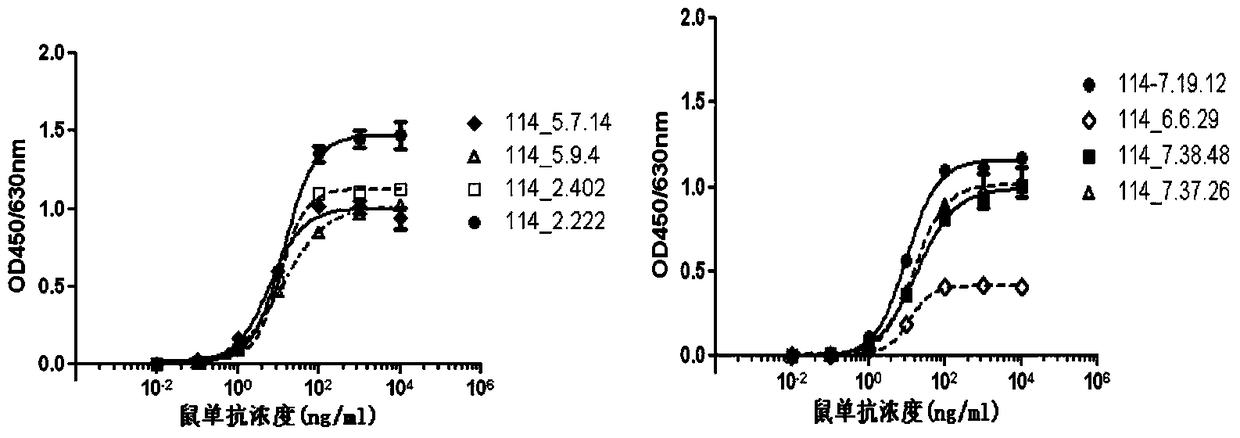
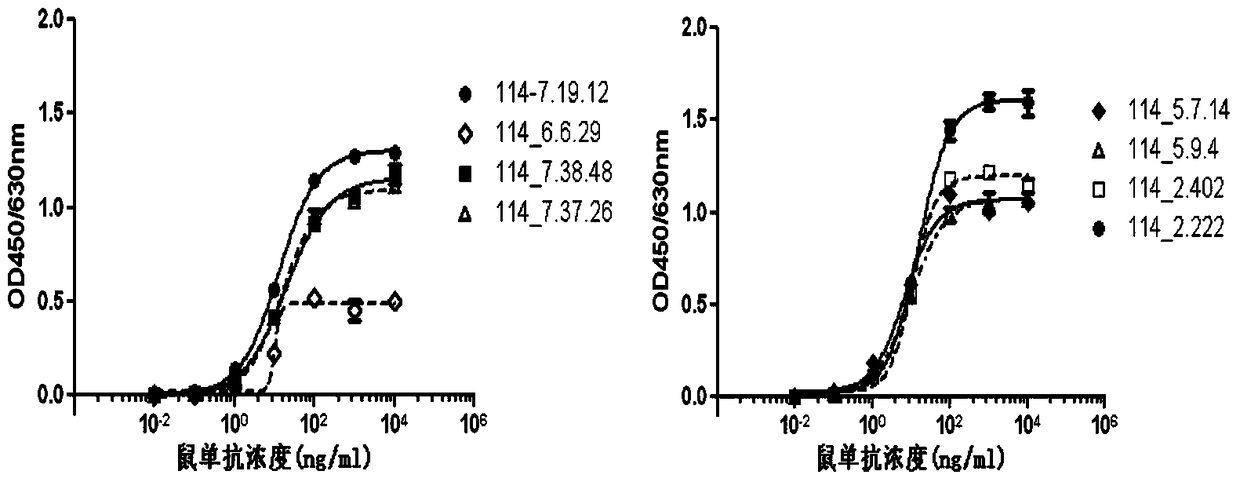
A kind of anti-human rank1 humanized antibody and its pharmaceutical composition and application
A humanized antibody, selected technology, applied in drug combinations, antibody mimics/scaffolds, antibodies, etc., can solve problems such as decreased antibody affinity and conformational changes in CDRs regions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1, the preparation of human source RANKL
[0152] By constructing a human RANKL expression vector and stably transfecting CHO cells, a cell line capable of stably and highly expressing human RANKL was screened. The cell line was cultivated on a large scale, the cell supernatant was collected, and purified by a nickel column to prepare human RANKL protein, which was used for mouse immunization, clone screening and functional identification in Examples 2, 3, 4, 5, 6 and 7.
[0153] CHO cells were purchased from Invitrogen Company, RAW264.7 cells were purchased from Shanghai Chinese Academy of Sciences Cell Bank, the expression vector was provided by our company, T4DNA ligase, protein molecular weight standard marker, restriction endonuclease, etc. were purchased from NEB Company; gel recovery kit was purchased from From Invitrogen; 302 medium, trypsin and FBS were purchased from Invitrogen; Phenylsepharose6FF-low sub, SP-Sepharose FF, Ni-NTA Sepharose FF gel we...
Embodiment 2
[0165] Embodiment 2, mouse immunization and titer determination
[0166] The antigen used for immunization (recombinant human RANKL) was from Example 1, and the BALB / c mice were purchased from Beijing Tonglihua Experimental Animal Technology Co., Ltd. Monoclonal antibody against RANKL was obtained by immunizing BALB / c mice several times. The way of immunization was plantar injection, and the immunization dose was 10 μg / 50 μl / mouse, 25 μl per foot. A total of 10 mice were immunized.
[0167] For the first immunization, mix 10 μg human RANKL with an equal volume of TiterMax (Sigma, Oakville, ON) for 10 subsequent immunizations. Mix 10 μg human RANKL with an equal volume of 100 μg alum (Sigma, Oakville, ON) and 10 μl pyrogen-free CpG-containing D-PBS without adjuvant . Immunization of BALB / c mice occurred on days 0, 5, 10, 15, 20, 25, 30, 35, 40 and 44, respectively, and four mice with the highest serum titers were taken for fusion on day 44.
[0168] After the fifth (20th ...
Embodiment 3
[0172] Example 3, Generation of anti-human RANKL mouse monoclonal
[0173] Immunize mice with CO 2 After euthanasia, the cervical vertebrae were dislocated, the lymph nodes were isolated, and the lymph nodes from different mice were combined. Put it into DMEM medium for grinding, collect the supernatant, centrifuge to obtain lymphocytes, and count them with a hemocytometer.
[0174] Wash the B cells obtained above, and mix them with non-secretory myeloma cells P3X63Ag8.653 (ATCC, Cat#CRL1580) at a ratio of 1:1, centrifuge the cell mixture at 800g, remove the supernatant gently, and add 2-4ml Pronase solution (CalBiochem, cat.#53702; 0.5mg / ml in PBS), the action does not exceed 2 minutes, add 3-5ml fetal bovine serum to terminate the enzyme reaction, add cell electrofusion solution ECFS (0.3M Sucrose, Sigma, Cat#S7903, 0.1mM MagnesiumAcetate, Sigma, Cat#M2545, 0.1mM Calcium Acetate, Sigma, Cat#C4705) Adjust the volume to 40ml, centrifuge, remove the supernatant, resuspend the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com