Replication type human adenovirus and application thereof
A human adenovirus, replicating technology, applied in the field of gene therapy, can solve the problems of lack of virus resources, lack of international recognition of clinical efficacy, and no oncolysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] A method for preparing a replicative human NBOV1901 oncolytic adenoviral vector, comprising the following steps:
[0078] S1) The left and right ends of the NBOV1901 genome were obtained by PCR amplification, connected to the ampicillin-resistant vector plasmid to obtain pT-NBOV1901 (L+R), and recombined with the NBOV1901 genome after linearization to obtain the genomic plasmid pNBOV1901;
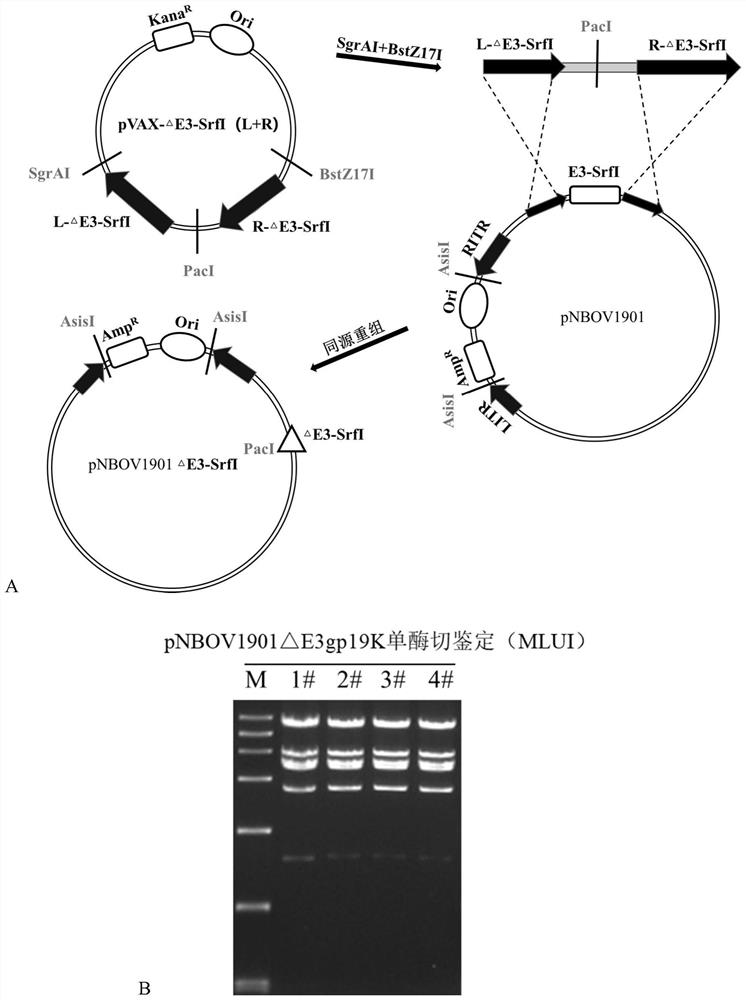
[0079] S2) The right and left arms from SrfI to the entire E3 region were obtained by PCR amplification, connected to the kanamycin-resistant vector plasmid, and recombined with PacI-digested NBOV1901 after linearization to obtain the genomic plasmid pNBOV1901 from which SrfI was removed to the entire E3 region △E3-SrfI;
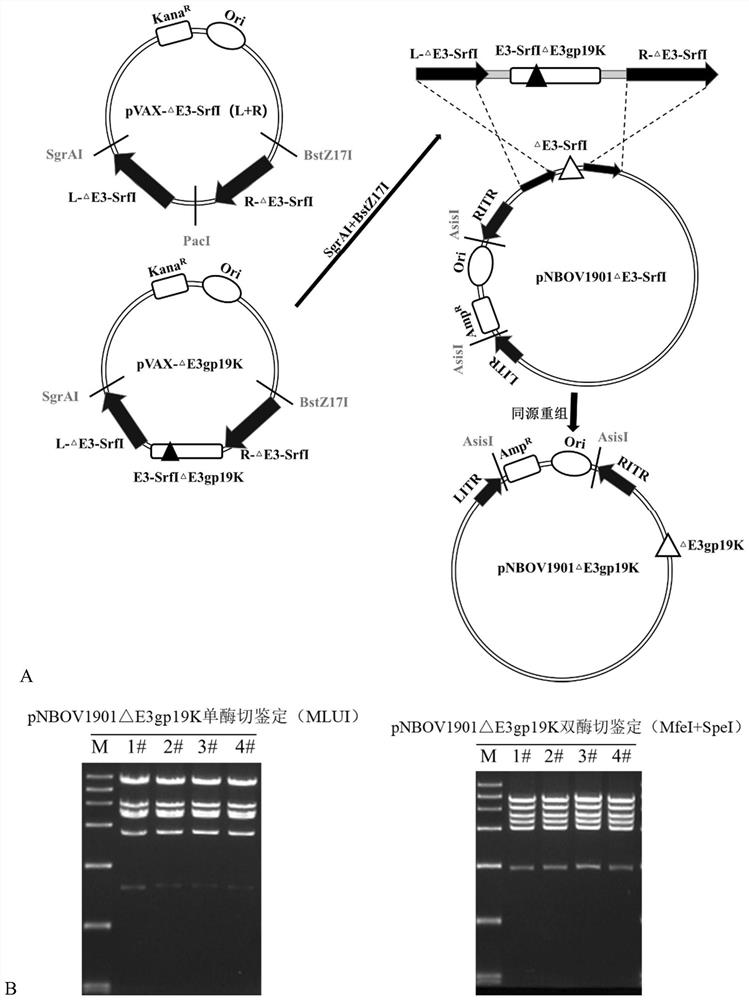
[0080] S3) Using the NBOV1901 genome as a template, the upstream fragment of E3gp19K was amplified by PCR: SrfI to E3gp19K, and the downstream fragment of E3gp19K: the region from E3gp19K to E3 contained the E3-14.7K gene fragment. The three fragments of the const...
Embodiment 1
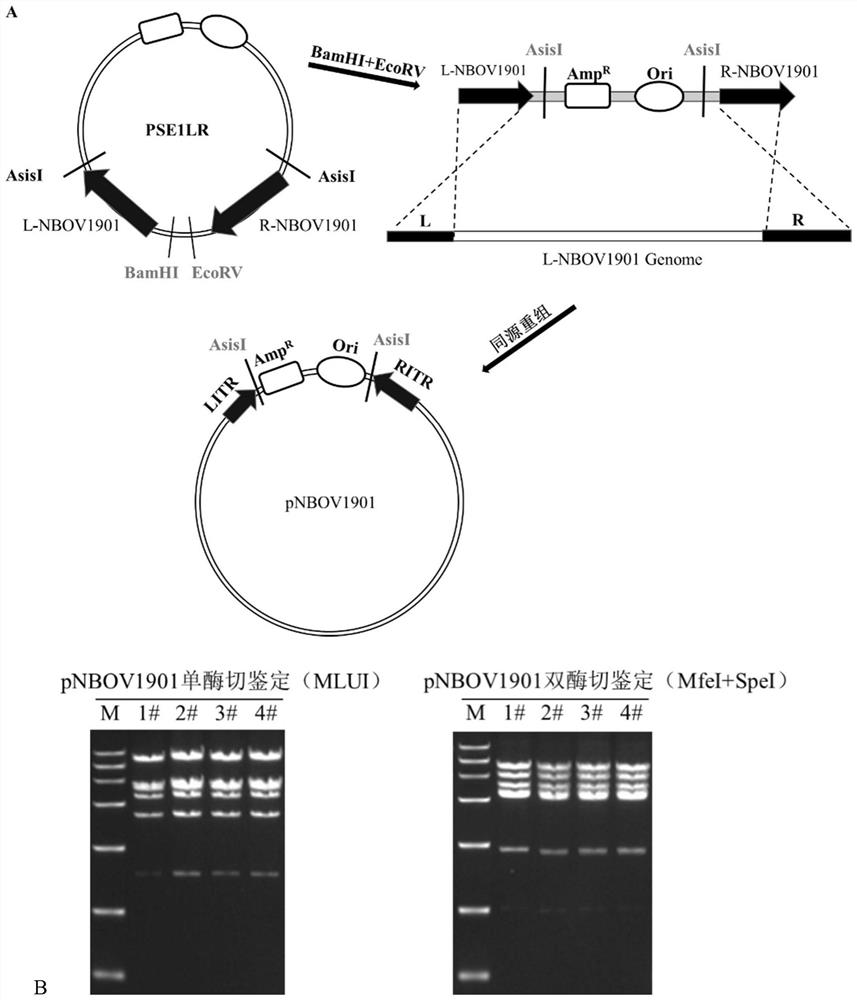
[0107] Example 1: Circularization of the NBOV1901 genome
[0108] 1. Construction of the shuttle plasmid pT-NBOV1901(L+R) for circularizing the NBOV1901 genome.
[0109] refer to figure 1 A, Using the genome of NBOV1901 as a template, the left arm (L-NBOV1901) and the right arm (R-NBOV1901) of the NBOV1901 genome were obtained by PCR.
[0110] L-NBOV1901 primer:
[0111] L-NBOV1901-F: gcgggatccgaattcttaatgcgatcgccatcatcaataataatacccttat (SEQ ID NO.: 1)
[0112] L-NBOV1901-R: tatctgcatgagcatgatgatatcctttgacccggaacgcgg (SEQ ID NO.: 2)
[0113] PCR conditions: 95°C, 3min; 95°C, 30s; 60°C, 30s; 72°C, 30s; cycles 28; 72°C, 5min;
[0114] R-NBOV1901 primer:
[0115] R-NBOV1901-F: ccgcgttccgggtcaaaggatatcatcatgctcatgcagata (SEQ ID NO.: 3)
[0116] R-NBOV1901-R: gaagcgagatcgaattcttagcgatcgccatcatcaataaatacctta (SEQ ID NO.: 4)
[0117] PCR conditions: 95°C, 3min; 95°C, 30s; 60°C, 30s; 72°C, 1min; cycles 28; 72°C, 5min;
[0118] 2. Construction of pNBOV1901.
[0119] pT-NBOV190...
Embodiment 2
[0120] Example 2: Knockout of genes from SrfI to E3 region, construction of pNBOV1901ΔE3_SrfI plasmid
[0121]1. Construction of the shuttle plasmid pVax-ΔE3_SrfI(L+R) for gene knockout from SrfI to E3 region.
[0122] refer to figure 2 A, Using the genome of NBOV1901 as a template, the left arm (L-ΔE3_SrfI) and right arm (R-ΔE3_SrfI) of the gene from SrfI to E3 region were obtained by PCR.
[0123] L-ΔE3_SrfI primer:
[0124] L-ΔE3_SrfI-F: gatatacgcgtgtatac cttcccaggatggcaccca (SEQ ID NO.: 5)
[0125] L-ΔE3_SrfI-R: gtaagtaatttattgtgtgtgtttatg ttaattaa ctgtgtgaccgctgctgt (SEQ ID NO.: 6)
[0126] PCR conditions: 95°C, 3min; 95°C, 30s; 60°C, 30s; 72°C, 30s; cycles 28; 72°C, 5min;
[0127] R-ΔE3 primer:
[0128] R-ΔE3_SrfI-F: acagcagcggtcacacag ttaattaa cataaacacacaataaattacttac (SEQ ID NO.: 7)
[0129] R-ΔE3_SrfI-R: ccgcccagtagaagcgccggtg ccgcccgttttaatttccatgtt (SEQ ID NO.: 8)
[0130] PCR conditions: 95°C, 3min; 95°C, 30s; 60°C, 30s; 72°C, 50s; cycles 28; 72°C, 5min; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com