CDK5 antigen epitope peptide and application thereof
An antigen epitope and antigen technology, applied in the field of molecular immunology, can solve the problem of insufficient immunogenicity of tumor-specific antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Prediction and synthesis of HLA-A0201-restricted CDK5 protein CTL epitope peptide
[0044] (1) Determination of the amino acid sequence of CDK5 protein:
[0045] By searching the CDK5 protein sequence in the Genbank database, a CDK5 protein sequence with the Genbank accession number: CAG33322.1 was obtained, which consists of 292 amino acid residues, as shown in SEQ ID No.1.
[0046] (2) Online prediction of CDK5 protein epitope peptide:
[0047] Using the Epitope Peptide Prediction Database
[0048] a: https: / / www-bimas.cit.nih.gov / cgi-bin / molbio / ken_parker_comboform and
[0049] b: http: / / www.syfpeithi.de / bin / MHCServer.dll / EpitopePrediction.htm)
[0050] The service provided is to make a preliminary prediction of the HLA-A0201-restricted CDK5 protein epitope peptide, select several epitope peptides with the highest scores, and then use the supermotif and quantitative motif scheme to modify the initially predicted CTL epitope peptide , to further increas...
Embodiment 2
[0060] Example 2: T2 cell epitope peptide binding affinity and stability experiments
[0061] The affinity and stability of HLA-A0201 and peptides were determined with the help of the characteristics of T2 cells.
[0062] (1) T2 cell affinity experiment
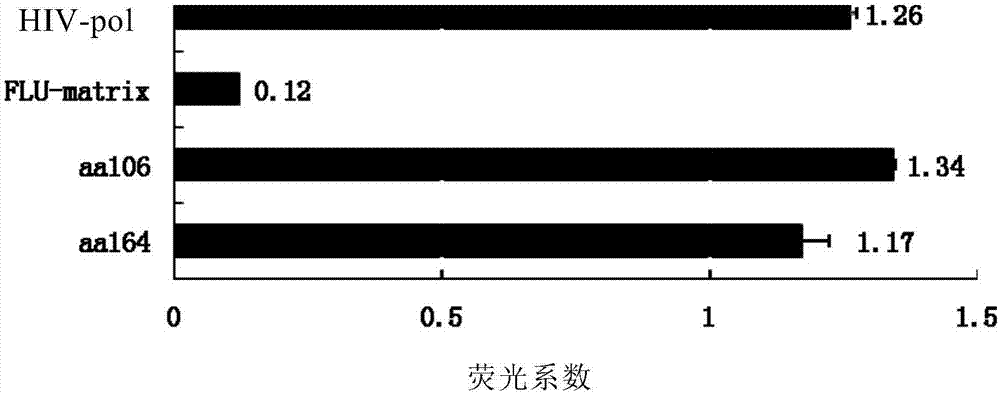
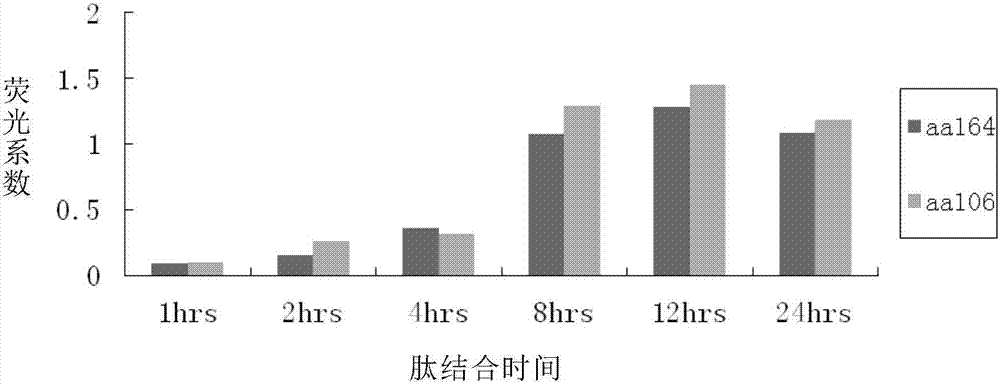
[0063] Collect T2 cells, centrifuge and wash three times with sterile PBS at 4°C, add serum-free 1640 medium, 2×10 5 Cells per well were seeded in a 24-well cell culture plate, and an experimental group and a control group were set up. Three secondary wells were repeated in each group. T2 cells stimulated without peptide were used as blank controls, and corresponding experimental peptides (aa106 and aa164) and control peptide (positive control: HIV pol peptide; negative control: influenza virus matrix protein peptide FLU-matrix) (final concentration 50uM), while adding β2 microglobulin (final concentration 2.5ug / ml). Place cells at 37 °C, 5% CO 2 Incubate in the incubator for 18 hours, collect the cells again, wash three t...
Embodiment 3
[0070] Example 3: Detection of pentamer-positive cytotoxic T cells corresponding to peptide aa106
[0071] The corresponding pentamer of peptide aa106 is based on the principle of double recognition of T cell activation. The heavy chain α and β2 microglobulins of MHC class I molecules are assembled in vitro by bioengineering technology, and combined with the antigenic epitope peptide to form a TCR specific Sexually bound monomers, and then five monomers are assembled together to form a pentamer.
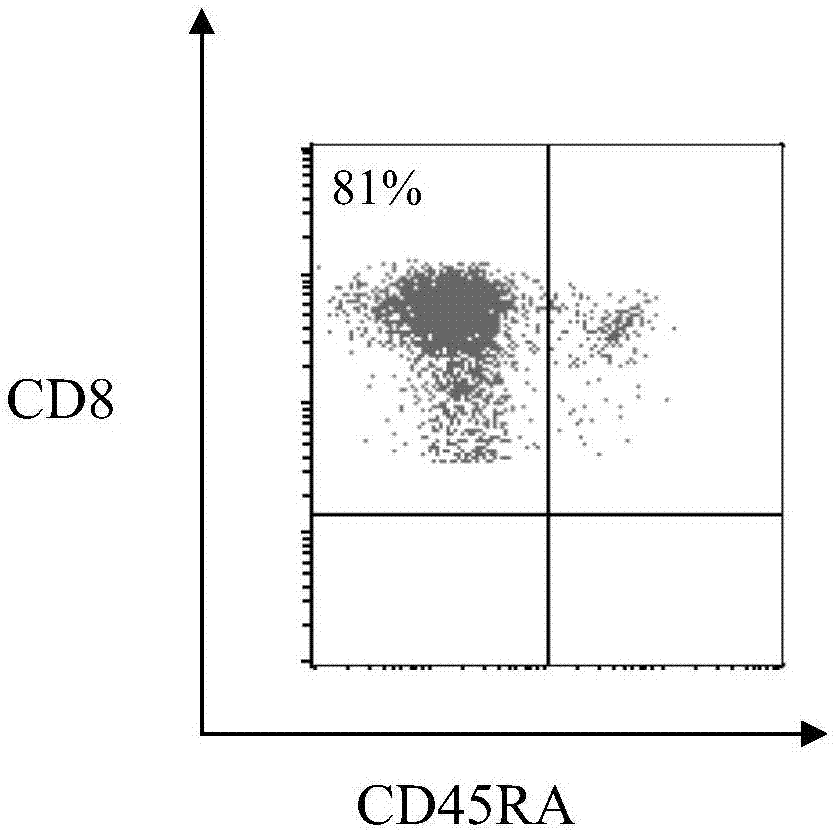
[0072] Two healthy donors and six patients with glioma who were diagnosed with non-remission were selected, and the CDK5 epitope peptide pentamer-positive CD8 in peripheral blood was detected. + number of cells. Collect 5ml of peripheral blood from healthy donors and patients with glioma diagnosed without remission, extract peripheral blood mononuclear cells with lymphocyte separation medium, count the cells and take 1×10 6 Dissolve in 100ul PBS, add 2ul peptide aa106 corresponding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com