Use of 2-phenyl-6-(1h-imidazol-1-yl)quinazoline for treating neurodegenerative diseases, preferably alzheimer's disease
A neurodegenerative, quinazoline technology for neurological diseases, antiviral agents, organic active ingredients, etc., that can address issues such as failure to reach primary endpoints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
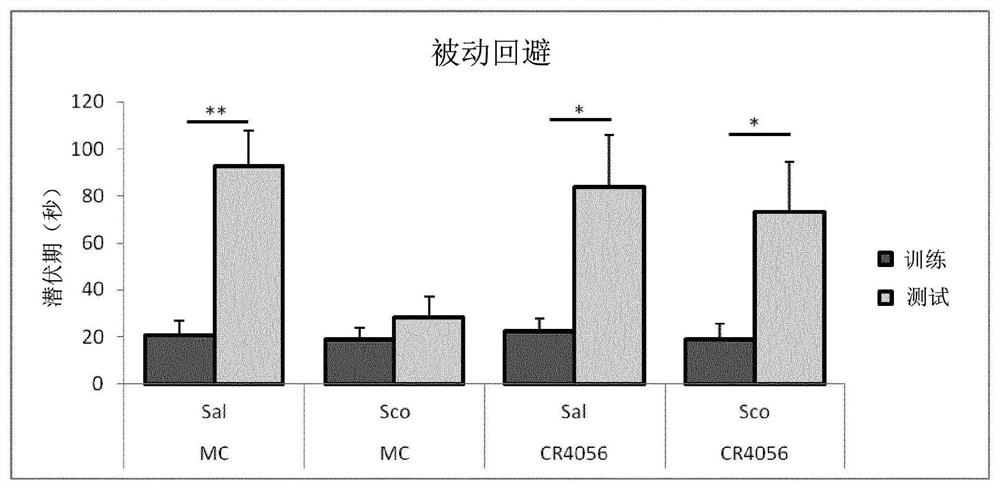
Examples
Embodiment 1
[0064] Example 1: Effect of 2-phenyl-6-(1H-imidazol-1-yl)quinazoline on inflammatory gene expression in vitro
[0065] method
[0066] These experiments used astrocytes, a human glioblastoma astrocytoma cell line U373 MG (Uppsala) model. in DMEM supplemented with 10% FBS at 37 °C and CO 2 Next, culture adherent cells. 72 h after plating, cells were treated with 2-phenyl-6-(1H-imidazol-1-yl)quinazoline (CR4056) (10 μM) prepared according to EP2066653 for 1 h, followed by the pro-inflammatory cytokine IL-1β (2ng / mL) stimulation for 6 and 24h.
[0067] At the end of the incubation period, total RNA was obtained and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The expression levels of COX-2, IL-1β, IL-6 and TNFα were assessed by RT-PCR analysis using the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems 7500 Fast Real-Time PCRSystem), using specific TaqMan assays, as Endogenous control, use 18S pre-...
Embodiment 2
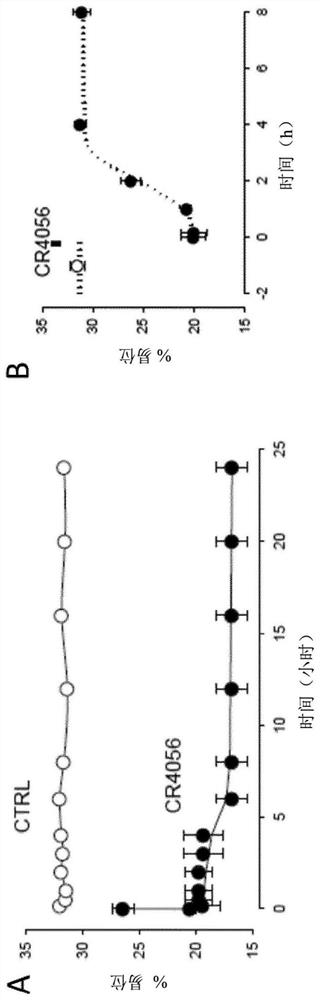
[0076] Example 2: In vitro effect of 2-phenyl-6-(1H-imidazol-1-yl)quinazoline (CR4056) on the translocation of PKCε to the plasma membrane in cultured neurons
[0077] method
[0078] Rat dorsal root ganglia (DRG) were obtained from freshly isolated spinous processes after careful removal of the nerve trunk and connective tissue. Larger ganglia were cut into 2-4 small pieces and incubated in 0.125% collagenase (Worthington, Freehold, NJ) in Dulbecco's Modified Eagle's Medium (DMEM) at 37°C For 1 hour, the medium contained 10% fetal bovine serum (FBS) plus 1% penicillin / streptomycin and 1% L-glutamine (Euroclone, Milan, Italy). After enzymatic digestion, ganglia were mechanically dissociated and placed in Petri dishes containing wells with glass-bottom coverslips (pre-coated with 10 μg / mL poly-L-lysine (poly-L-lysine) and 20 μg / ml laminin, Sigma Aldrich, Milan, Italy), neurons were plated at a density such that neurons covered about 30% of the coverslip surface in a single la...
Embodiment 3
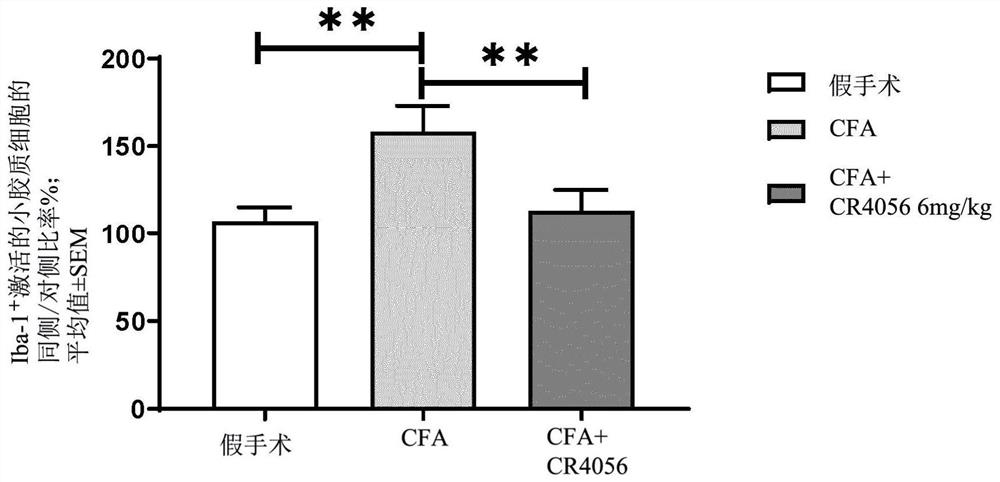
[0084] Example 3: In vivo effect of 2-phenyl-6-(1H-imidazol-1-yl)quinazoline (CR4056) on microglial activation in the CFA inflammation model
[0085] method
[0086] Microglial activation was assessed by immunofluorescence staining, and in the complete Freund's adjuvant (CFA) model, the expression of ionized calcium-binding adapter molecule 1 (Iba-1) was measured in the ipsilateral L5 spinal cord.
[0087] Induce unilateral inflammation by injecting 100 μL of CFA (1 mg / mL, diluted 1:1 with saline) into the phalanx of the rat's right hind paw.
[0088]At 72 hours after CFA, CR4056 (6 mg / kg, os) was administered, and 90 minutes later, the animal was deeply anesthetized with excess urethane (1.5 g·kg-1, i.p.), and then transcardially perfused with 250 mL of 0.9% 1 % heparin in saline (5000 UI·mL-1 ), followed by 500 mL of 10% formalin (ie 4% paraformaldehyde, Bio-Optica Spa, Milan, Italy). Spinal cord L5 segments were harvested, fixed overnight at 4°C, and embedded in paraffin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com