Method for separating double-bond-comprising straight-chain hydrocarbon in which all hydrogen atoms have been substituted with fluorine atoms or chlorine atoms
A separation method, hydrogen atom technology, applied in the field of straight chain hydrocarbons, can solve the problems of high vapor pressure, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
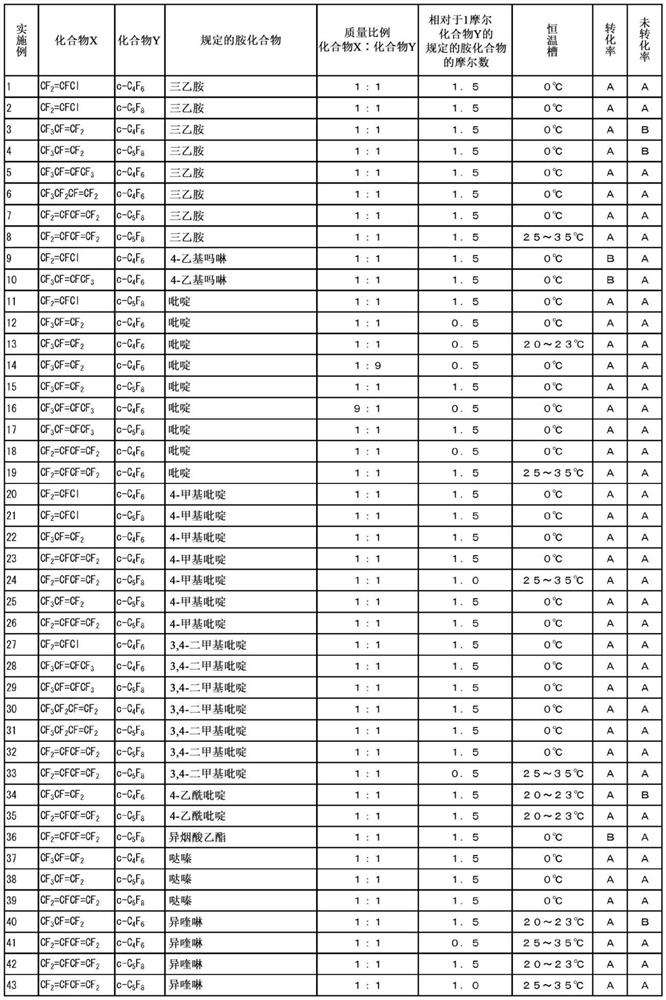
Examples
Embodiment 12
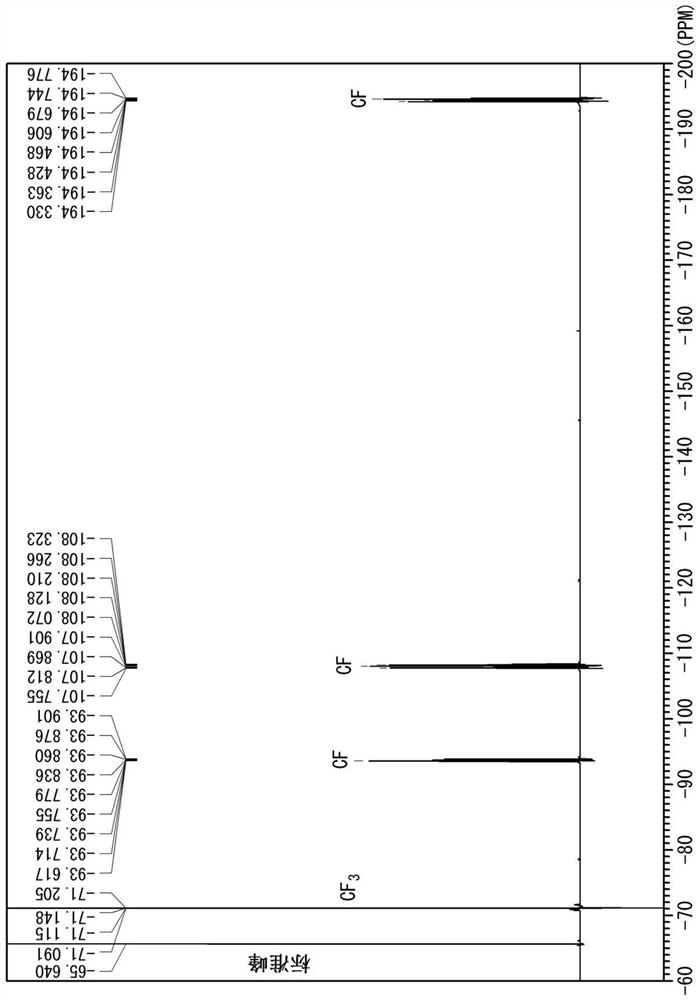
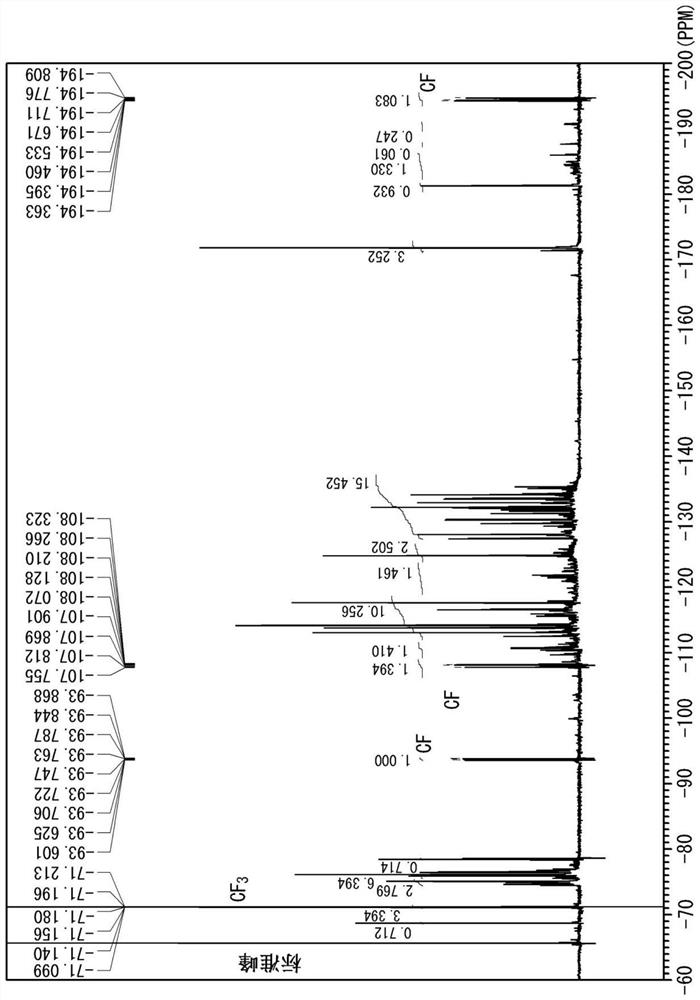
[0095] The results of the fluorine NMR measurement of Example 12 are shown in figure 1 .
[0096] figure 1 It is the measurement result of the compound inside the second pressure vessel. The corresponding peaks are from CF 3 CF=CF 2 peak, c-C was not detected 4 f 6 The peak (-122.2ppm (CF 2 ) and -131.0ppm (CF)).
[0097] figure 2 It is the measurement result of the compound inside the first pressure vessel. c-C not detected 4 f 6 peaks, presumed to be from CF 3 CF=CF 2 Peaks other than the peaks from the product.
[0098] Based on the integrated values of the peaks of Compound X, Compound Y, and the reaction product in the fluorine NMR measurement, the respective ratios in the pressure vessel were calculated, and the mass of the compound Y was calculated from the previously calculated mass of the residual component and the mass of the collected gas. Conversion rate, unconverted rate of compound X.
[0099] The conversion rate of the compound Y is the ratio o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com