CD3/CD28/DLL4 magnetic bead and preparation method and application thereof
A technology of magnetic beads and antibodies, applied in the biological field, can solve the problems of easy drug resistance of tumors, toxic and side effects, etc., and achieve the effects of controllable product quality, obvious effect, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
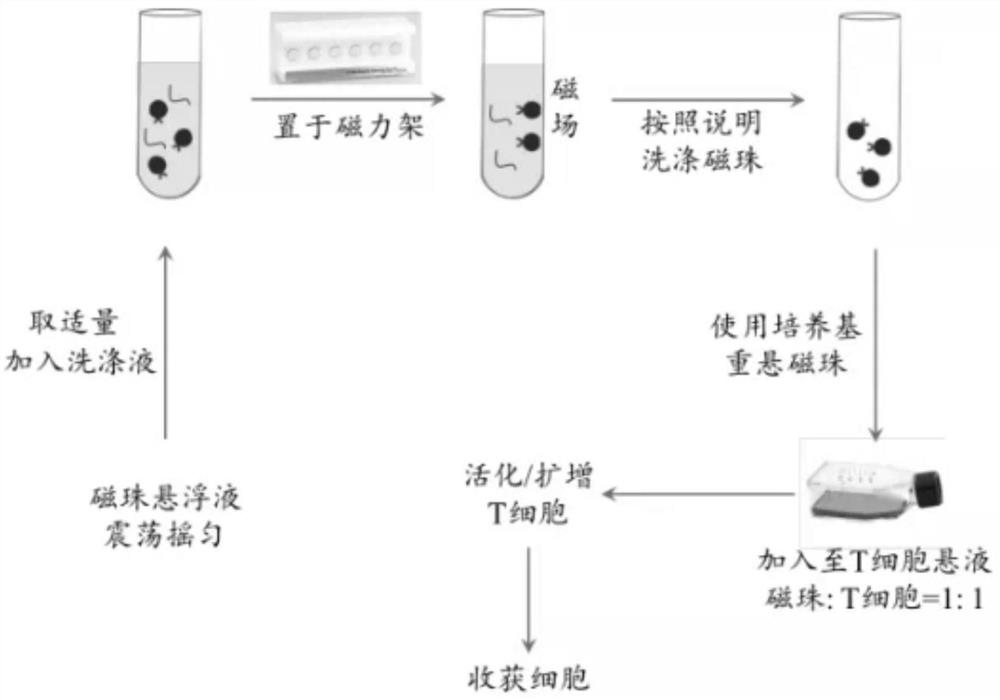
[0048] Embodiment 1: A method for amplifying and producing human effector CD8 + Preparation method and application of CD3 / CD28 / DLL4 magnetic beads for T lymphocytes;
[0049] 1) Preparation of CD3 / CD28 / DLL4 functional magnetic beads: Take 1*10^6~5*10^6 magnetic beads, wash the magnetic beads twice with MEST buffer (1 mL each time), add the pre-configured EDC solution , Vortex and mix well, incubate at 25°C for 20-60 minutes, mix the activated magnetic beads with the following protein solution and incubate: 25 μg anti-human-CD3 antibody solution + 25 μg anti-human-CD28 antibody + 10 μg DLL4 recombinant protein solution at 25°C Continue Incubate for 2-4 hours, avoid the magnetic beads from settling during the incubation period, then block the magnetic beads with MEST solution containing BSA for 0.5-2 hours, the blocking temperature is 25°C, and finally wash the magnetic beads 3 times with TBST (1 mL each time), and then store them After washing the magnetic beads for 3 times, s...
Embodiment 2
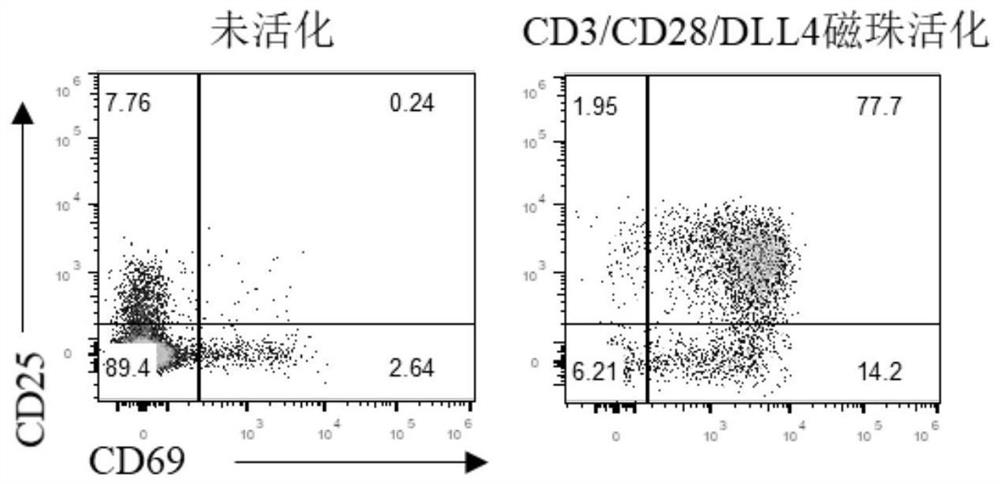
[0052] Example 2: CD3 / CD28 / DLL4 functional magnetic bead activation efficiency test;
[0053] 1) Take 0.2M purified T lymphocytes, add T cell special medium to adjust the total volume of cell suspension to 200 μL;
[0054] 2) Add the CD3 / CD28 / DLL4 magnetic beads prepared in Example 1, and wash the magnetic beads once with 1mL F-PBS before use;
[0055] 3) Spread the mixture of cells and magnetic beads on a 96-well plate and place in CO at 37°C 2 Cultivate overnight in an incubator;
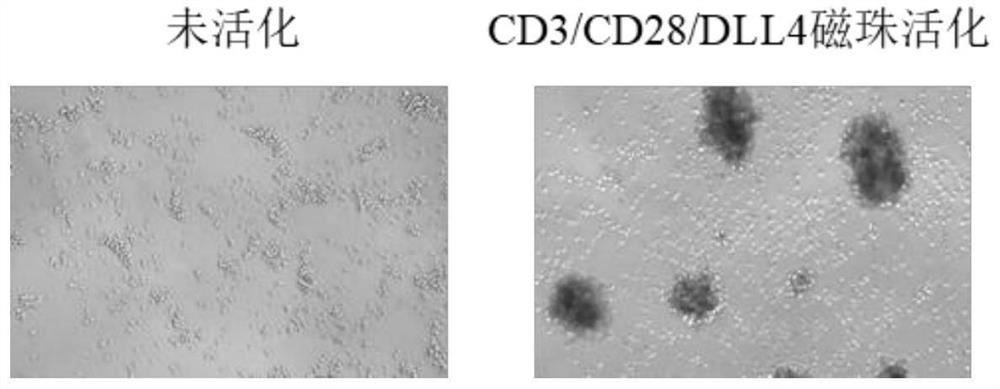
[0056] 4) The next day, observe the state of the cells and the formation of clones under a microscope, and the results of the photos are attached figure 2 shown. figure 2 It shows that activated by CD3 / CD28 / DLL4 magnetic beads, the magnetic beads bind to form cell-magnetic bead complexes around the cells, and T lymphocytes have obvious proliferating clone populations, while the morphology of cells in the wells without magnetic beads does not change , and there is no proliferating clonal popu...
Embodiment 3
[0058] Example 3: Verification of cell expansion after activation of T lymphocytes by CD3 / CD28 / DLL4 magnetic beads;
[0059] 1) The T lymphocytes collected in Example 2 and activated overnight by CD3 / CD28 / DLL4 magnetic beads were kept in 37°C CO 2 Cultivated in an incubator;
[0060] 2) Take out the cells on the third day of culture, count them, take 0.1M cells and resuspend them in 1.5mL T cell special medium, spread them on a new 24-well plate and continue to culture them in a CO2 incubator at 37°C;
[0061] 3) Take out the cells on the seventh day of culture, count them, take 0.1M cells and resuspend them in 1.5mL T cell special medium, spread them on a new 24-well plate and continue to store them in 37°C CO 2 Cultivated in an incubator;
[0062] 4) Take out the cells on the tenth day of culture, count them, and calculate the cell expansion multiples. The expansion results are as attached Figure 4 shown; Figure 4 showed that CD3 / CD28 / DLL4 magnetic beads activated cult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com