Synthesis process of phenylbutyrin
A technology of glyceryl phenylbutyrate and synthesis process, which is applied in the preparation of carboxylate, the preparation of organic compounds, organic chemistry and other directions, can solve the problems such as difficulty in controlling the reaction end point, long reaction time, complicated solvent, etc., and reduce impurities The effect of producing, improving conversion rate and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
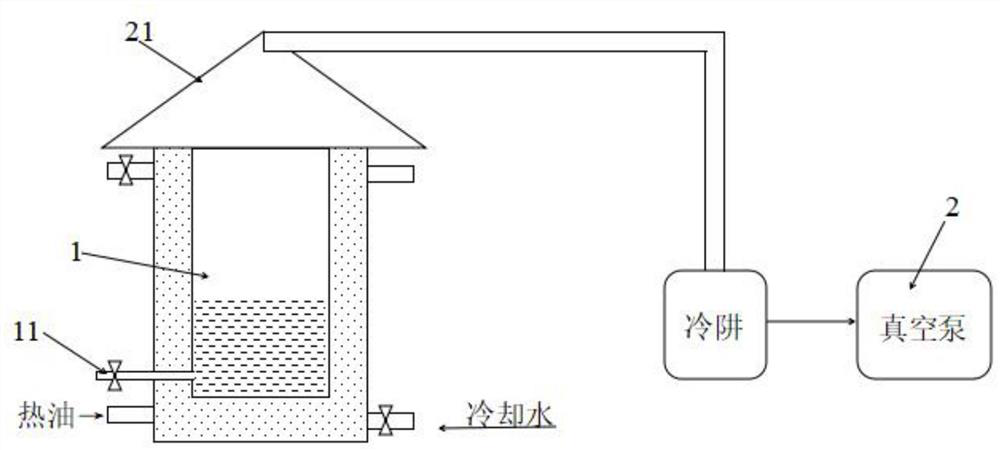
[0047] Add 187g of glycerol (2.03mol) to a 2.5L reactor, heat up to 120°C, add 1000g (6.09mol) of phenylbutyric acid in batches, stir until completely melted, and add about 20g of strong acid catalytic resin amberlyst15. Raise the temperature to 160°C, react at 0.8KPa for 2h; then rapidly raise the temperature to 220°C, continue to react at 0.8KPa for 3h, take samples to measure the impurity content of the components in the reactor, and react for 3.5h, the measured impurity content is less than 1.0 %. Cool the reactor rapidly to terminate the reaction. Let the reaction solution stand at room temperature, centrifuge at 10,000*g for 30 minutes, remove catalyst and solid impurities, and obtain the stock solution of glyceryl phenylbutyrate, which is purified by three-stage nylon 66 filter membrane with a pore size of 0.8 μm, 0.4 μm, and 0.2 μm. , to obtain the product.
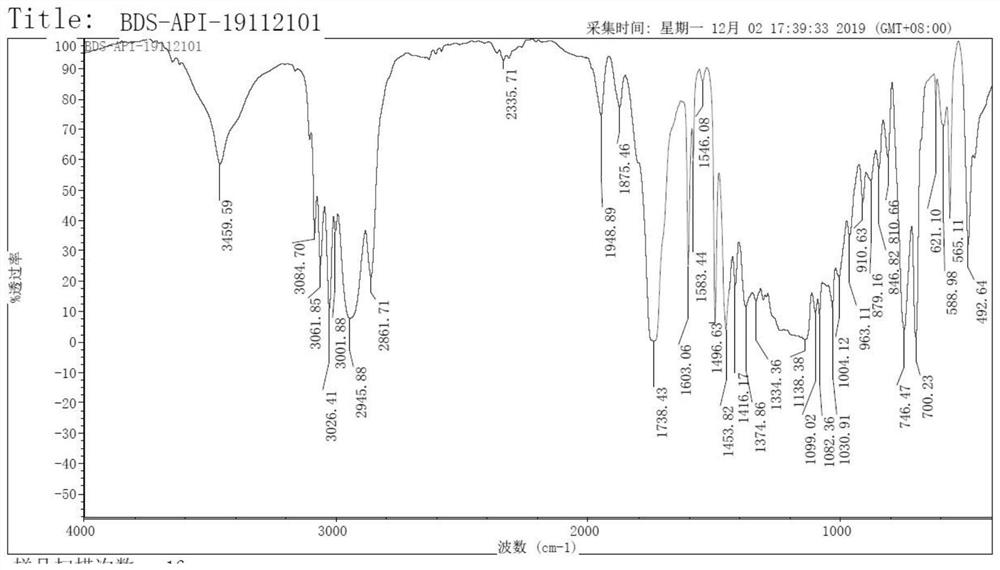
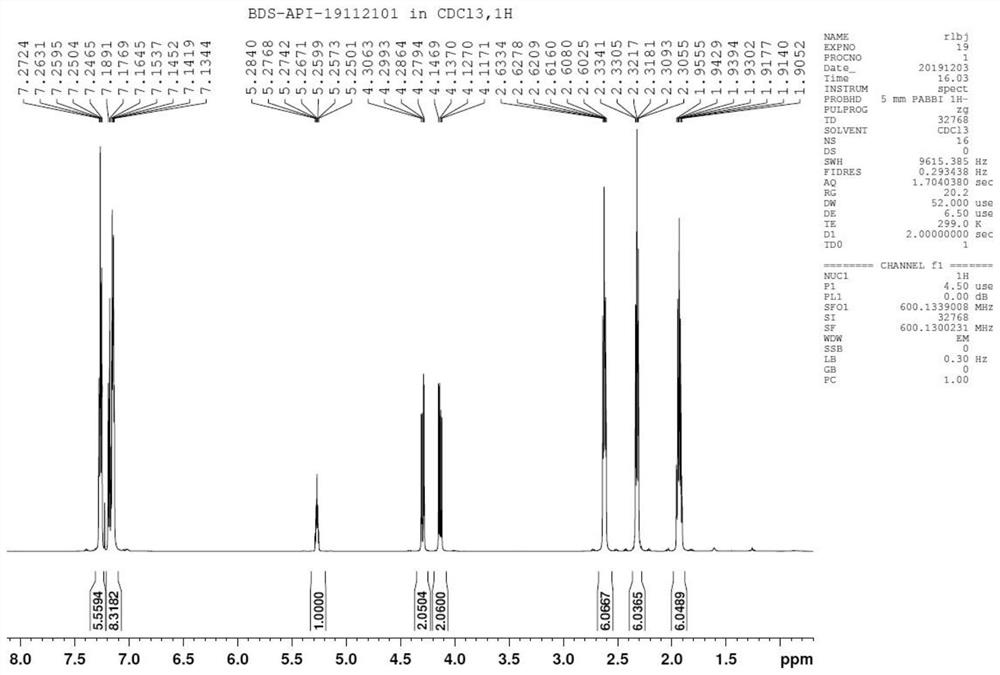
[0048] Validation and analysis of the final product, including:
[0049] (1) Infrared absorption spectrum (I...
Embodiment 2
[0078] Add 187g of glycerol (2.03mol) to a 2.5L reactor, raise the temperature to 122°C, add 1000g (6.09mol) of phenylbutyric acid in batches, stir until completely melted, and add about 20g of strong acid catalytic resin amberlyst15. Raise the temperature to 150°C, react at 0.6KPa for 2.5h; then rapidly raise the temperature to 225°C, continue to react at 0.6KPa for 3h, take samples to measure the impurity content of the components in the reactor, and react for 4h, the measured impurity content is less than 1.0 %. Cool the reactor rapidly to terminate the reaction. Let the reaction solution stand at room temperature, centrifuge at 10,000*g for 30 minutes, remove catalyst and solid impurities, and obtain the stock solution of glyceryl phenylbutyrate, which is purified by three-stage nylon 66 filter membrane with a pore size of 0.8 μm, 0.4 μm, and 0.2 μm. , to obtain pure phenylbutyric acid glyceride; according to the HPLC condition test of embodiment 1, its purity reaches 98....
Embodiment 3
[0080] Add 187g of glycerol (2.03mol) to a 2.5L reactor, heat up to 120°C, add 1000g (6.09mol) of phenylbutyric acid in batches, stir until completely melted, and add about 20g of strong acid catalytic resin amberlyst15. Raise the temperature to 155°C, react at 0.4KPa for 2.5h; then rapidly raise the temperature to 220°C, continue to react at 0.4KPa for 3h, take samples to measure the impurity content of the components in the reactor, and react for 4h, the measured impurity content is less than 1.0 %. Cool the reactor rapidly to terminate the reaction. Let the reaction solution stand at room temperature, centrifuge at 10,000*g for 30 minutes, remove catalyst and solid impurities, and obtain the stock solution of glyceryl phenylbutyrate, which is purified by three-stage nylon 66 filter membrane with a pore size of 0.8 μm, 0.4 μm, and 0.2 μm. , to obtain pure glyceryl phenylbutyrate; according to the HPLC conditions of Example 1, its purity reaches 98.57%, and its purity is low...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com