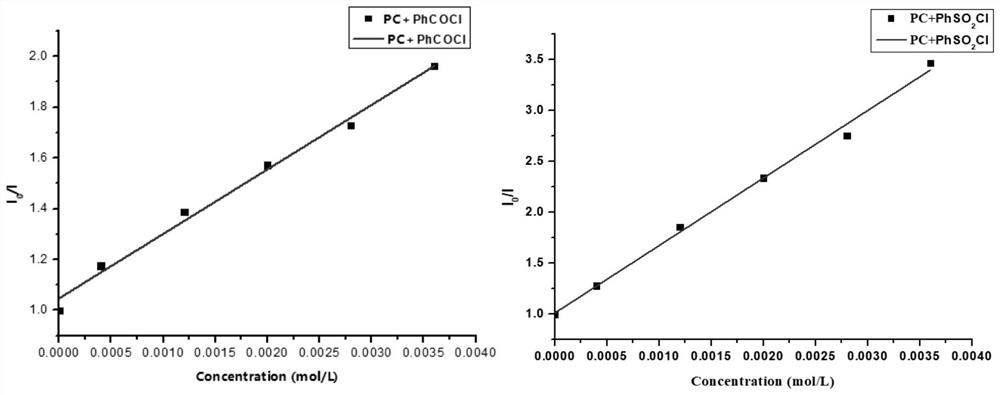
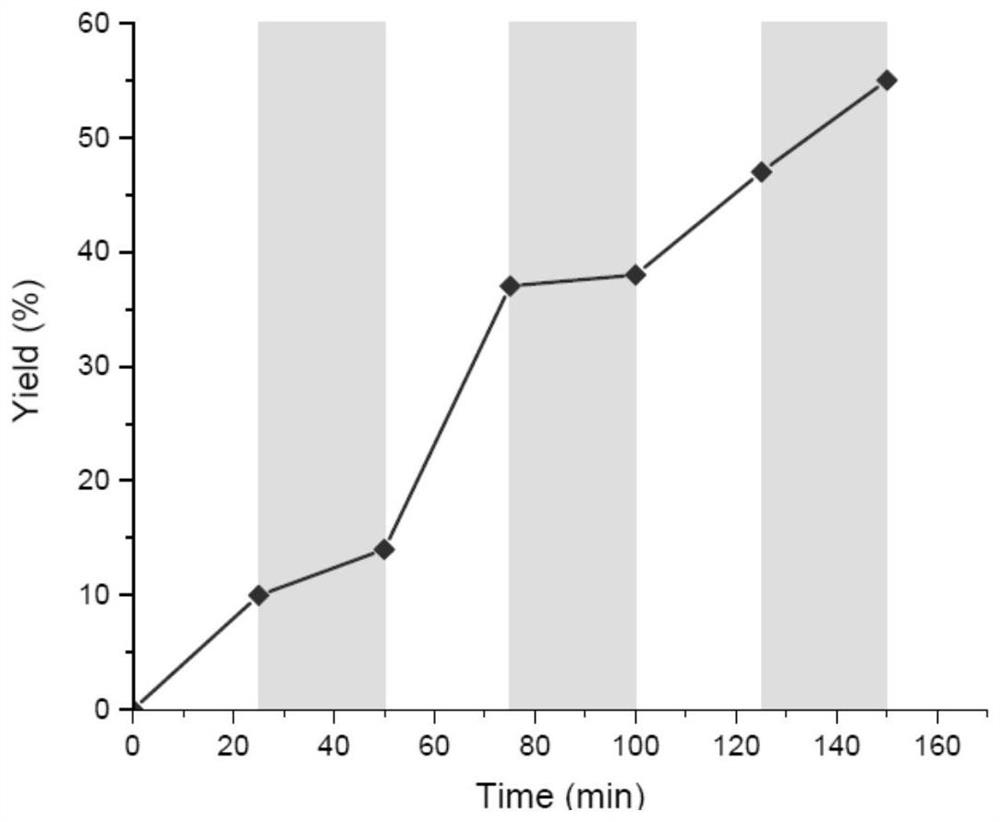
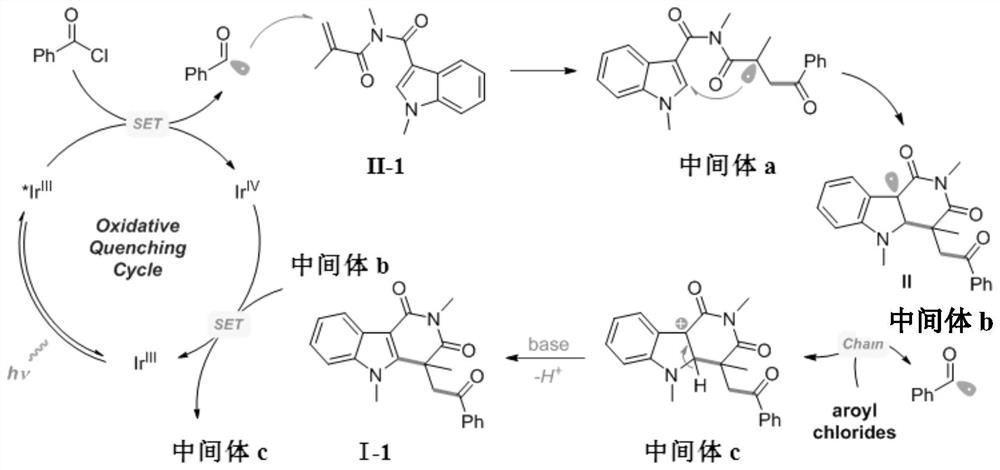
Method for preparing indolotetrahydropyridinedione and derivative thereof through photo-initiated free radical cascade reaction and product
A technology of tetrahydropyridinedione and indole derivatives, which is applied in the field of organic compound preparation and can solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The compound (2,4,5-trimethyl-4-(2-oxo-2-phenylethyl)-4,5-dihydro-1H-pyrido[4,3-b]indole-1,3 for preparation of structural formula I-1 (2H)-dione), the preparation method is as follows:
[0036] (1) Add 0.2mmol R 1 for -CH 3 , R 2 for -CH 3 Indole derivatives (structural formula II-1), 0.4mmol of 2,6-lutidine (2,6-lutidine) and 1.0mmol of acid chloride (R 3 -Cl, where R 3 for ) into a round-bottomed flask equipped with a magnetic stirrer, adding the face formula three (2-phenylpyridine) iridium (fac-Ir(ppy) 3 ) (3mol%, relative to the addition of indole derivatives), vacuumize and backfill with argon (three times);
[0037] (2) Inject 2 mL of degassed and dried organic solvent (CH 2 Cl 2 ), at room temperature, under an argon atmosphere (argon balloon), irradiate with a blue LED lamp with a power of 9W, stir and react for 4h to obtain a mixture (the reaction is monitored by thin-layer chromatography, and the indole derivative is reacted until the solution is n...
Embodiment 2
[0042] The compound (2,4,5-trimethyl-4-(2-oxo-2-(p-tolyl)ethyl)-4,5-dihydro-1H-pyrido[4,3-b] of preparation structure I-2 indole-1,3(2H)-dione), the preparation method is as follows:
[0043] R in Example 1 3 for The acid chloride is replaced by R 3 for acid chloride, and the rest were reacted according to the method in Example 1 to prepare 56.9 mg of a compound of structural formula I-2 (white solid, yield 76%).
[0044] 1 H NMR (600MHz, Chloroform-d) δ8.41–8.31(m,1H),7.76(d,J=8.0Hz,2H),7.34–7.28(m,3H), 7.24(d,J=8.0Hz, 2H), 4.34(d, J=18.0Hz, 1H), 4.10(d, J=18.0Hz, 1H), 3.83(s, 3H), 3.42(s, 3H), 2.41(s, 3H), 1.77( s, 3H). 13 C NMR (151MHz, Chloroform-d) δ195.0, 176.3, 162.4, 146.7, 144.7, 138.4, 133.2, 129.4, 128.1, 124.8, 123.4, 122.6, 121.5, 109.2, 104.5, 47.0, 44.0, 31.9, 26.6 .
[0045] HRMS(ESI)calcd.for C 23 h 22 N 2 o 3 [M+Na] + :397.1523, found 397.1532.
Embodiment 3
[0047] The preparation structural formula is the compound (4-(2-(4-(tert-butyl)phenyl)-2-oxoethyl)-2,4,5-trimethyl-4,5-dihydro-1H-pyrido[4, 3-b] indole-1,3(2H)-dione), the preparation method is as follows:
[0048] R in Example 1 3 for The acid chloride is replaced by R 3 for acid chloride, and the rest were reacted according to the method in Example 1 to prepare 58.3 mg of a compound of structural formula I-3 (white solid, yield 70%).
[0049] 1 H NMR (600MHz, Chloroform-d) δ8.36 (dd, J = 5.9, 2.5Hz, 1H), 7.80 (d, J = 8.2Hz, 2H), 7.45 (d, J = 8.2Hz, 2H), 7.34 –7.28(m,3H),4.35(d,J=18.0Hz,1H),4.10(d,J=18.0Hz,1H),3.84(s,3H),3.42(s,3H), 1.77(s, 3H), 1.34(s, 9H). 13 C NMR (151MHz, Chloroform-d) δ195.1, 176.3, 162.4, 157.6, 146.7, 138.4, 133.1, 125.7, 124.8, 123.4, 122.6, 121.5, 109.2, 104.4, 47.0, 44.0, 35.2, 32.0, 21.0, .
[0050] HRMS(ESI)calcd.for C 26 h 28 N 2 o 3 [M+Na] + : 439.1992, found 439.1993.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com