Method for preparing acesulfame potassium
A technology of acesulfame potassium and acesulfame potassium, which is applied in the field of preparation of acesulfame potassium, to achieve the effect of increasing the color value of the product, reducing the content of organic impurities, and reducing the decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
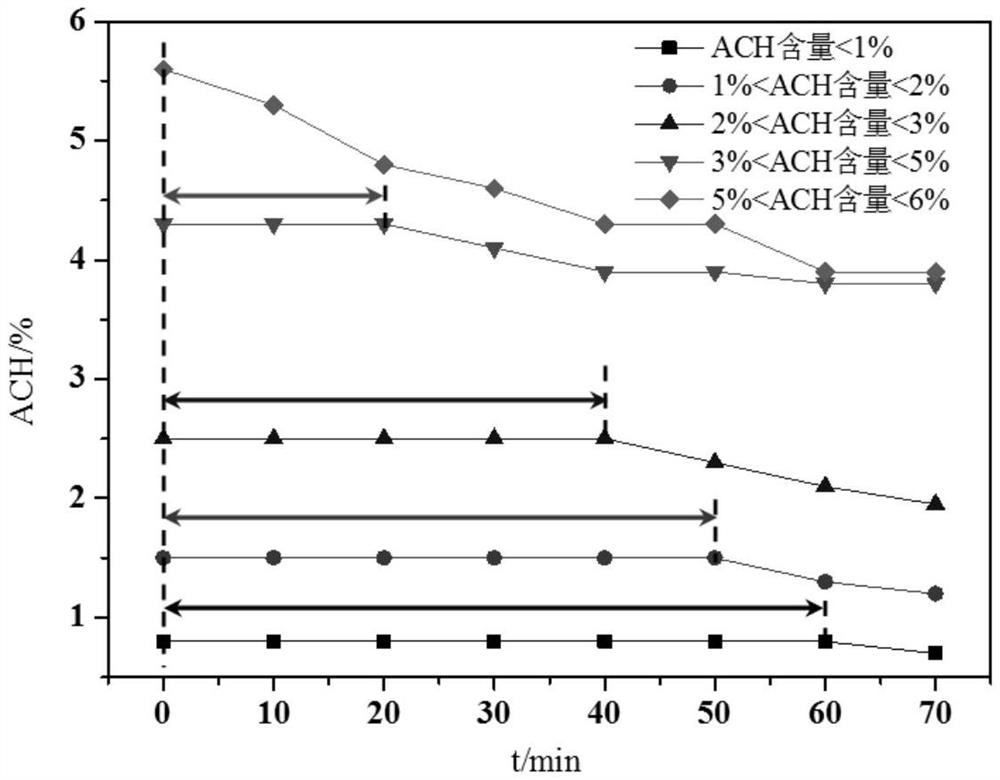
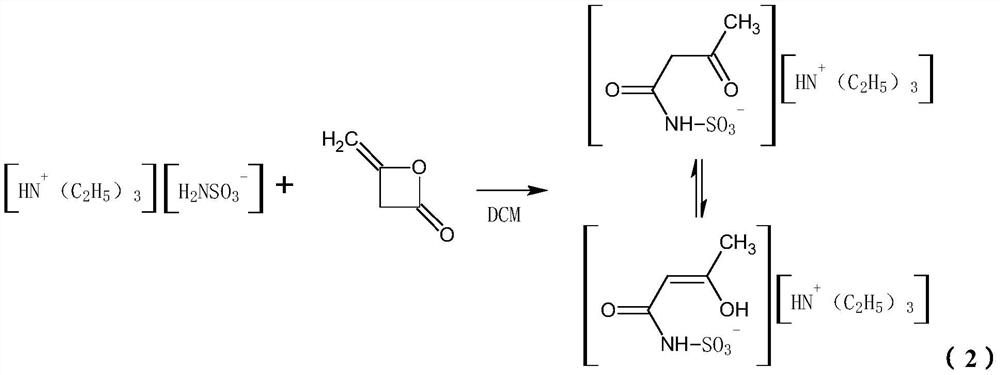
[0063] Using dichloromethane as solvent, sulfamic acid is neutralized with triethylamine, and the prepared solution is acylated with diketene to obtain DKA (acetoacetamide-N-sulfonate) solution. 20% SO 3 Solution and 35% DKA solution are introduced into the input end of the cyclization reactor, and the material after the cyclization reaction is directly sprayed into the hydrolysis reactor at the outlet of the reactor to carry out a hydrolysis reaction with a certain amount of water; after the hydrolysis reaction is completed, the sulfuric acid in the acid layer The content of ACH in the acid layer is 45%, and the content of ACH in the acid layer is 2.2%; the residence time of the hydrolyzate in the hydrolysis reactor is 40min, and the content of ACH in the acid does not change. After extraction and neutralization, a crude acesulfame potassium composition was obtained with a color value of 8.3 Hazen, and then refined acesulfame potassium product was obtained through evaporation...
Embodiment 2
[0065] Using dichloromethane as solvent, sulfamic acid is neutralized with triethylamine, and the prepared solution is acylated with diketene to obtain DKA (acetoacetamide-N-sulfonate) solution. 30% SO 3 Solution and 25% DKA solution are introduced into the input end of the cyclization reactor, and the material after the cyclization reaction is directly sprayed into the hydrolysis reactor at the outlet of the reactor to carry out a hydrolysis reaction with a certain amount of water; after the hydrolysis reaction is completed, the sulfuric acid in the acid layer The content of ACH in the acid layer is 60%, and the content of ACH in the acid layer is 2.8%; the residence time of the hydrolyzate in the hydrolysis reactor is 25 minutes, and the content of ACH in the acid does not change. After extraction and neutralization, a crude acesulfame potassium composition was obtained, with a color value of 8.5 Hazen, and then evaporated, concentrated, decolorized, and recrystallized to ob...
Embodiment 3
[0067] Using dichloromethane as solvent, sulfamic acid is neutralized with triethylamine, and the prepared solution is acylated with diketene to obtain DKA (acetoacetamide-N-sulfonate) solution. 40% SO 3 solution and 30% DKA solution are introduced into the input end of the cyclization reactor, and the material after the cyclization reaction is directly sprayed into the hydrolysis reactor at the outlet of the reactor to carry out a hydrolysis reaction with a certain amount of water; after the hydrolysis reaction is completed, the sulfuric acid in the acid layer The content of ACH in the acid layer is 50%, and the ACH content in the acid layer is 2.5%; the residence time of the hydrolyzate in the hydrolysis reactor is 35 minutes, and the ACH content in the acid does not change. After extraction and neutralization, a crude acesulfame potassium composition was obtained, with a chromaticity value of 8.1 Hazen, and then evaporated, concentrated, decolorized, and recrystallized to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color price | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com