Pentacyclic triterpenes in treatment of vitiligo
A technology of eucalyptol and composition, applied in the field of pentacyclic triterpenoids for treating vitiligo, can solve problems such as side effects, expensive treatment methods, addiction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
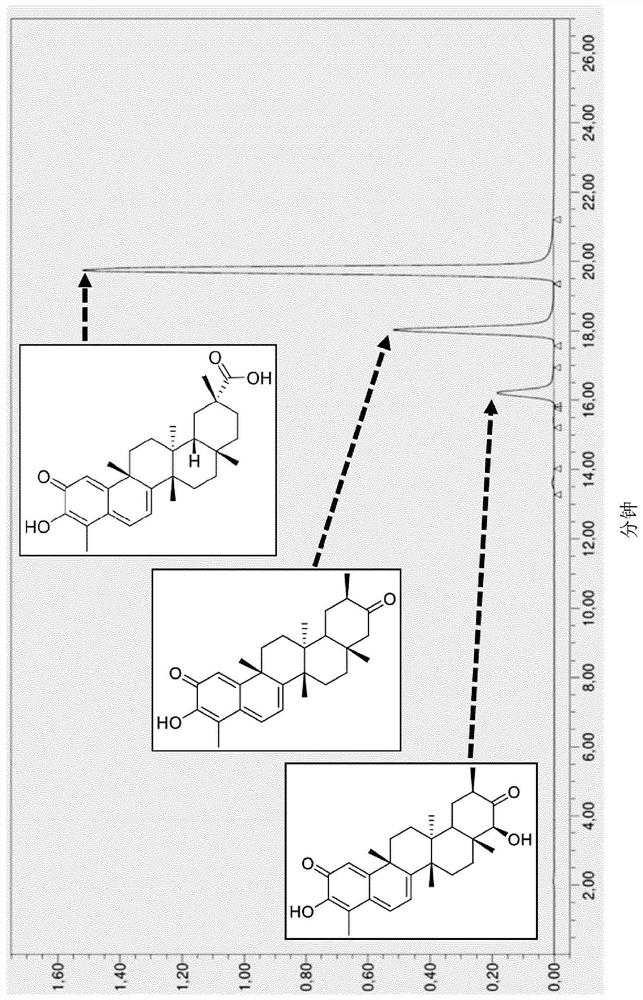
[0150] Example 1: Obtaining Tripterygium wilfordii plant cell culture extract CCV enriched in pentacyclic triterpenes
[0151] The cultivation was carried out in a Sartorius Stedim Biotech (Germany) wave reactor (volume 5 L). The reactor was inoculated with Tripterygium wilfordii cell suspension from Erlenmeyer flasks (media composition, parameters as mentioned above). The decoy was performed with a decoy mixture (methyl jasmonate and chitin) after reaching the maximum biomass after about 17 days under continuous agitation. The culture was stopped after 15 days of induction. Most of the biomass was recovered by filtering the cell suspension through a nylon filter (20-50 μm). Approximately 1925 g of biomass was recovered from the 5 L suspension. The biomass was extracted with ethyl acetate (or isopropyl acetate) at a ratio of 2:1 (volume:weight) to biomass weight (here 3850 mL solvent for 1925 g biomass). The biomass / solvent mixture is then physically extracted by sonicat...
Embodiment 2
[0153] Example 2: Anti-Inflammation and Anti-MMP-9 of Two Extracts and Purified Molecules Duelin A, Duelin B, and Cherendol active
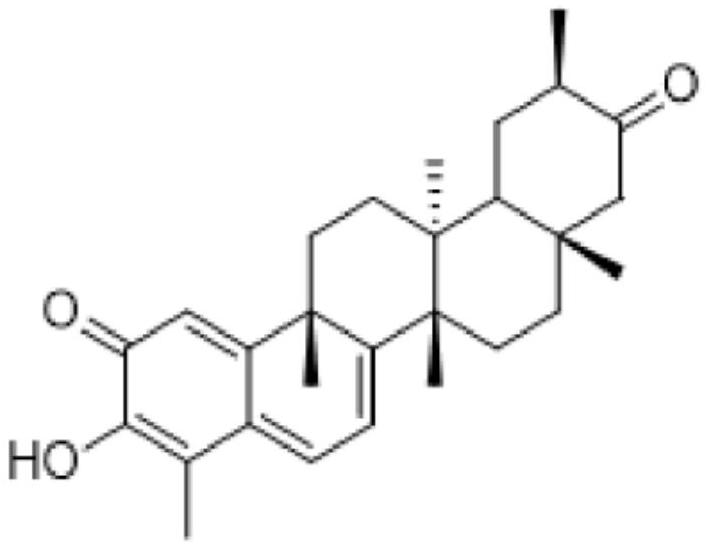
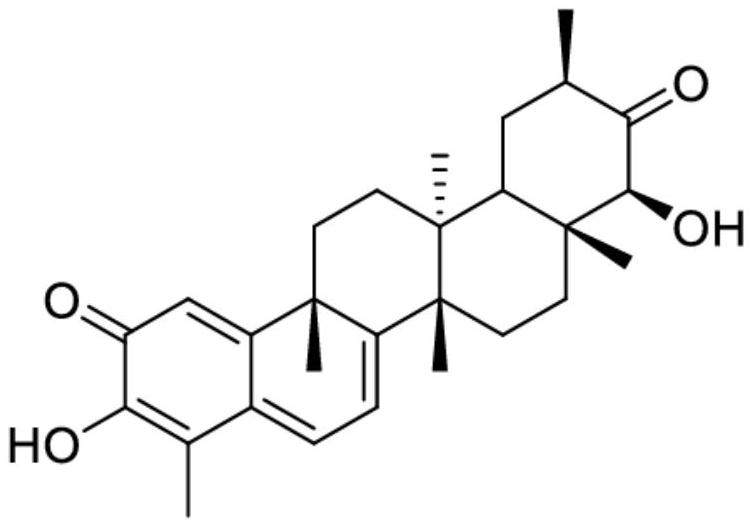
[0154] Two different extracts, derived from plant cell cultures, were tested in this example. Extract 2 was obtained according to Example 1, and Extract 1 was obtained from the same culture using a different inducement, ie methyl jasmonate and acetylsalicylic acid were used instead of methyl jasmonate and chitin. Extracts 1 and 2 are expressed as % of each triterpene component (Ducocin A: TA, Duelaccin B: TB, Celostrol: CEL) based on the total amount of TA, TB and CEL in the extract, see Table 1 below.
[0155] [Table 1]
[0156]
[0157] Extract stock solutions (dry weight of TA, TB and CEL) were quantified in μg / mL by HPLC (relative to standards). Test products were dissolved in DMSO (stock solution). For TA and TB, three concentrations were tested, 45, 135 and 405 ng / mL. For celandol, three concentrations were also tested, 15, 45 a...
Embodiment 3
[0186] Example 3: Antioxidant Activity of Two Extracts and Purified Molecules Duelin A, Duelin B and Cherendol
[0187] Oxidative stress is one of the possible reasons for the development of vitiligo. The purpose of this experiment was to measure the antioxidant activity of each pentacyclic triterpene and two extracts and compare it with vitamin C, a well-known positive control. The intube method used was adapted from the ORAC (Oxygen Radical Antioxidant Capacity) assessment method described by Davalos et al. (J Agric Food Chem 52(1):48-54, 2004). The ORAC index can evaluate the antioxidant capacity of each compound tested in Example 2. This test allows the evaluation and classification of the various compounds tested according to their antioxidant capacity against peroxyl radicals. AAPH (2,2'-Azobis-2-methyl-propionamidine, dihydrochloride) is a source of peroxy radicals. This test was used to simulate the kinetics of free radical degradation of fluorescein. This result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com