Method for recovering high-purity lithium carbonate by causticizing and freezing lithium precipitation mother liquor to remove mirabilite
A technology for sinking lithium mother liquor and lithium carbonate, applied in chemical instruments and methods, lithium carbonate;/acid carbonate, inorganic chemistry, etc., can solve the problems of large evaporation, high cost, and high processing cost, and achieve The effect of cheap raw materials, low cost and less raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
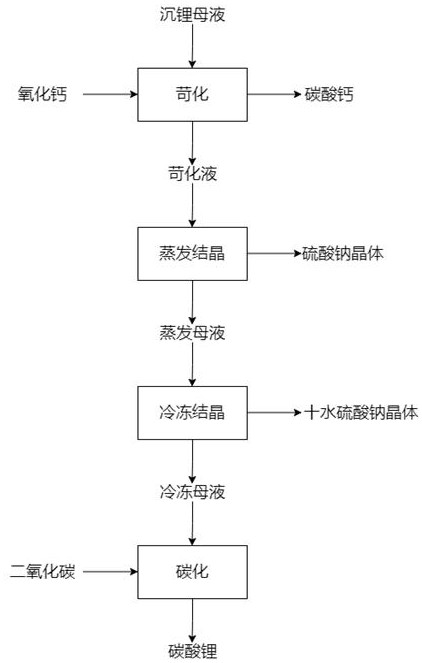
[0028] A method for recovering high-purity lithium carbonate by causticizing, freezing, and removing Glauber's salt from lithium-precipitating mother liquor, comprising the following steps: S1. The content is 2.3g / L, the carbonate ion content is 13g / L, the sodium ion content is 70g / L, and the sulfate ion content is 150g / L. Add calcium oxide containing 1.1-1.3 times the molar amount of carbonate ions. The specific amount of lithium sinking mother liquor is 5L, and the amount of calcium oxide added is 73g. After calcium oxide is added, the pH of the solution is 12.2, causticizing and stirring for 2.5 hours, and the temperature is 50 ℃, calcium carbonate precipitate and causticizing solution are obtained after filtration. S2. Concentration by evaporation: Evaporate and concentrate the causticizing solution obtained in step S1 to a solution specific gravity of 1.2, and then perform solid-liquid separation to obtain a concentrated solution (lithium ion concentration of 6.5 g / L) and...
Embodiment 2
[0030] A method for recovering high-purity lithium carbonate by causticizing, freezing, and removing Glauber's salt from lithium-precipitating mother liquor, comprising the following steps: S1. The content is 2.5g / L, the content of carbonate ion is 15g / L, the content of sodium ion is 75g / L, and the content of sulfate ion is 160g / L. Add calcium oxide containing 1.1-1.3 times the molar amount of carbonate ions. The specific amount of lithium sinking mother liquor is 5L, and the amount of calcium oxide added is 91g. After calcium oxide is added, the pH of the solution is 12.5 and the reaction is causticized and stirred for 3 hours. Calcium carbonate precipitate and causticizing solution are obtained after filtration. S2. Evaporation and concentration: the causticizing solution obtained in step S1 was evaporated and concentrated to a specific gravity of 1.23, followed by solid-liquid separation to obtain a concentrated solution (lithium ion concentration of 6.6 g / L) and sodium sul...
Embodiment 3
[0032]A method for recovering high-purity lithium carbonate by causticizing, freezing, and removing Glauber's salt from lithium-precipitating mother liquor, comprising the following steps: S1. The content is 2.7g / L, the carbonate ion content is 20g / L, the sodium ion content is 80g / L, and the sulfate ion content is 170g / L. Add calcium oxide containing 1.1-1.3 times the molar amount of carbonate ions. The specific amount of lithium sinking mother liquor is 5L, and the amount of calcium oxide added is 116g. After calcium oxide is added, the pH of the solution is 12.7, causticizing and stirring for 3 hours, and the temperature is 70°C , After filtration, calcium carbonate precipitate and causticizing solution are obtained. S2. Evaporation and concentration: the causticizing solution obtained in step S1 was evaporated and concentrated to a specific gravity of 1.25, followed by solid-liquid separation to obtain a concentrated solution (lithium ion concentration of 6.8 g / L) and sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com