Method for measuring concentration of furoate in human plasma through HPLC-MS/MS
A blood plasma and concentration technology, applied in the field of analysis, can solve problems such as low detection limit, achieve stable baseline, eliminate matrix effect, and improve recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
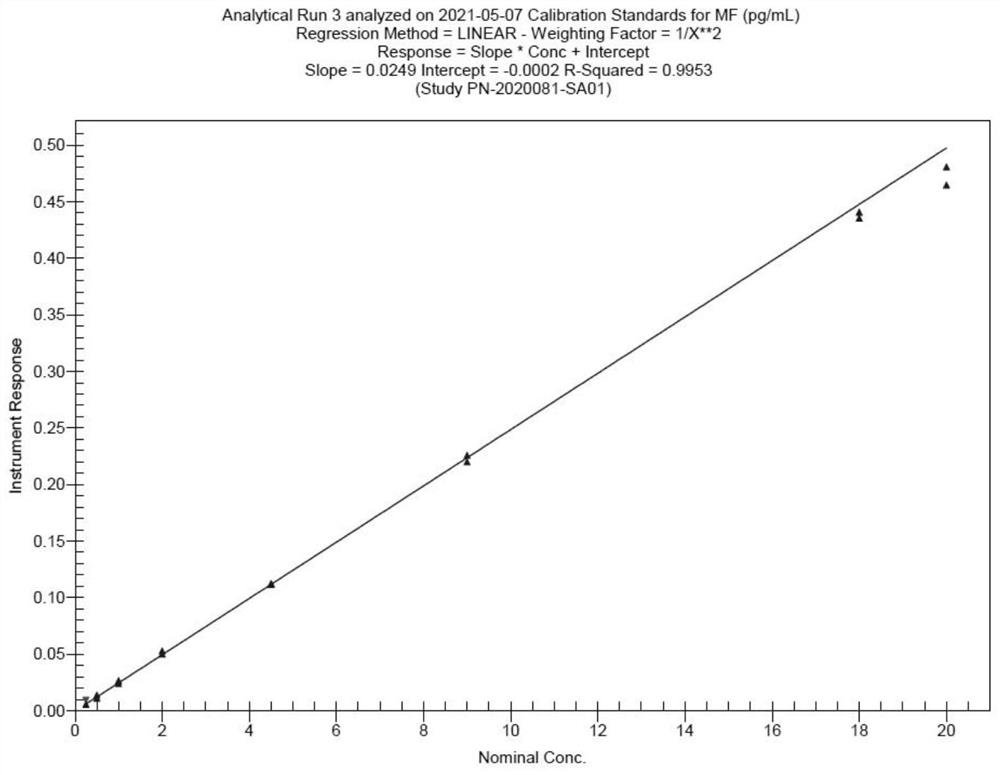
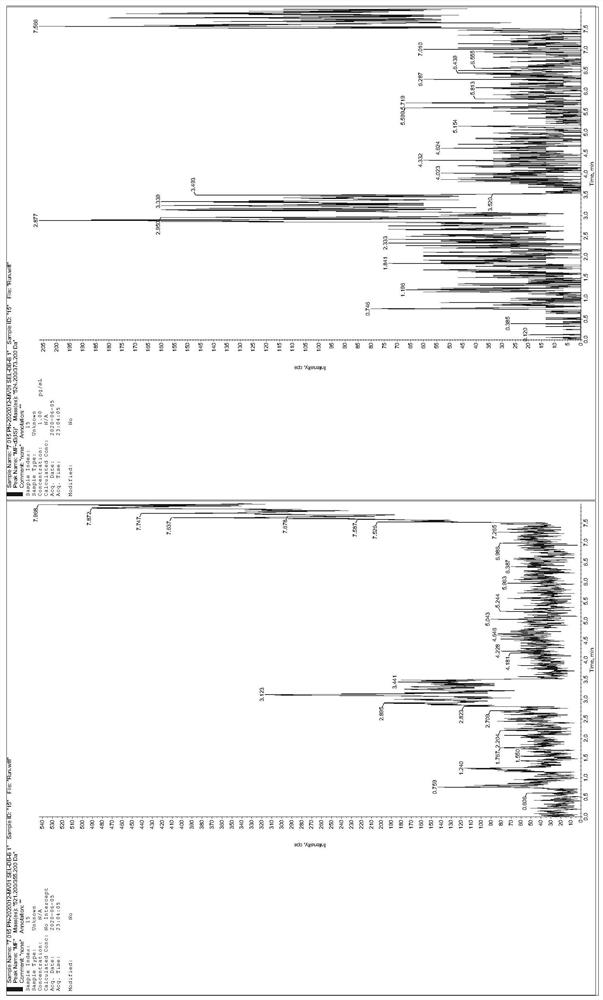
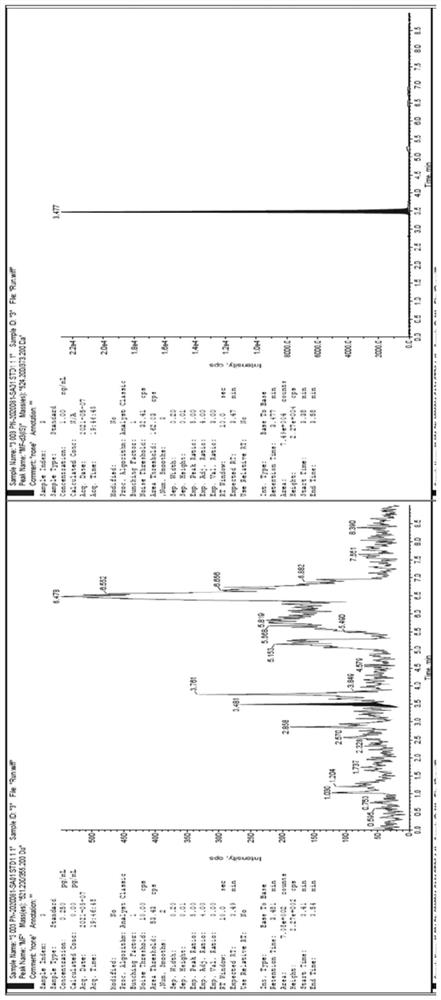
[0041] Establishment of an HPLC-MS / MS method for the determination of mometasone furoate concentration in human plasma:
[0042] Preparation of standard and quality control working solutions: Two analysts weighed about 3 mg of mometasone furoate standard substance, added appropriate amount of methanol solution, vortexed, ultrasonically dissolved, and prepared a standard substance stock with a concentration of 0.500 mg / mL liquid, marked MF-Stock. All stock solutions were stored in a refrigerator at 2-8°C for later use; the prepared stock solutions were diluted step by step with 50% acetonitrile, and the specific dilution concentrations were shown in Table 1 below:
[0043] Table 1-1 Mometasone furoate standard curve working solution preparation table
[0044]
[0045]
[0046] Note: The above preparation operations can be adjusted according to actual needs, and the final concentration remains unchanged.
[0047]Table 1-2 Preparation table of mometasone furoate quality c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com