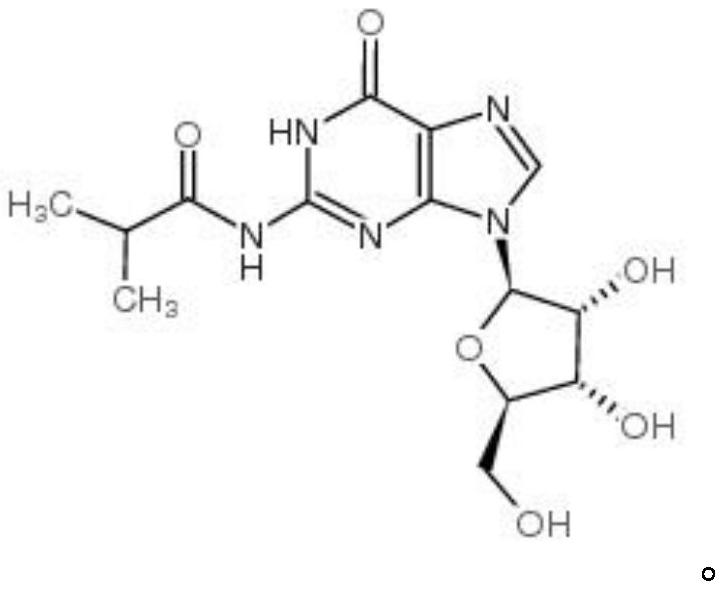
Purification method of N-isobutyryl guanosine
A technology of isobutyryl guanosine and isobutyryl guanosine is applied in the field of purification of N-isobutyryl guanosine, can solve the problems of low product purity, large content of by-products, difficult purification and the like, and achieves high purity, Simple protection and deprotection process and avoidance of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The purification process of Embodiment 1 of the present invention includes the following steps:
[0050] Step S1, under argon protection, mixed the guanine nucleoside and (BOC) 2O in a molar ratio of 1: 4.2, add water to completely dissolve, react, TLC monitors the guanine nucleoside, stopped the reaction, and then reacts The fluid concentrates to obtain a guanine nucleoside with a BOC protecting group.
[0051]Step S2, mixing step S1 with a BOC protecting group of guanine nucleoside, isobutyl bromide, and dichloromethane, and add sodium hydroxide to react, TLC monitors the guanine nucleoside with BOC protecting group. After the disappearance, the reaction was stopped, and the reaction solution was concentrated under reduced pressure, and purified by the silica gel chromatography column to obtain an N-isobutylglikamide reaction liquid with a BOC protective group.
[0052] Step S3, 20% silica gel in which 20% silica gel, 70 ~ 80 ° C, obtained to step S2, 70 to 80 ° C, to rem...
Embodiment 2
[0056] The purification process of Embodiment 2 of the present invention includes the following steps:
[0057] Step S1, under argon protection, mixed the guanine nucleoside and (boc) 2O at 1: 4.8 molar ratio, add water to completely dissolve, react, TLC monitor the guanine nucleoside completely disappeared, and then reacts The liquid is concentrated and purified, and then purified by silica gel chromatography to give a guanine nucleoside with a BOC protecting group.
[0058] Step S2, mixing step S1 with a BOC protecting group of guanine nucleoside, isobutyl bromide, and dichloromethane, and add sodium hydroxide to react, TLC monitors the guanine nucleoside with BOC protecting group. After the disappearance, the reaction was stopped, and the reaction solution was concentrated under reduced pressure, and purified by the silica gel chromatography column to obtain an N-isobutylglikamide reaction liquid with a BOC protective group.
[0059] Step S3, 18% silica gel in which 18% silica ...
Embodiment 3
[0063] The purification process of Embodiment 3 of the present invention includes the steps of:
[0064] Step S1, under argon protection, mix the guanine nucleoside and (Boc) 2O at 1: 4.4 molar ratio, add water to completely dissolve, react, TLC monitors the guanine nucleoside, stop the reaction, then the reaction The liquid is concentrated and purified, and then purified by silica gel chromatography to give a guanine nucleoside with a BOC protecting group.
[0065] Step S2, mixing step S1 with a BOC protecting group of guanine nucleoside, isobutyl bromide, and dichloromethane, and add sodium hydroxide to react, TLC monitors the guanine nucleoside with BOC protecting group. After the disappearance, the reaction was stopped, and the reaction solution was concentrated under reduced pressure, and purified by the silica gel chromatography column to obtain an N-isobutylglikamide reaction liquid with a BOC protective group.
[0066] Step S3, a 15% silica gel, 70 ~ 80 ° C, obtained by he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com