Ionizable lipid molecule, preparation method thereof and application of ionizable lipid molecule in preparation of lipid nanoparticles
An ionic and lipid-based technology, applied in the biological field, can solve the problems of not being able to fully exert the efficacy of mRNA-LNP preparations, achieve excellent mRNA carrier performance, and improve the effect of translation and expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
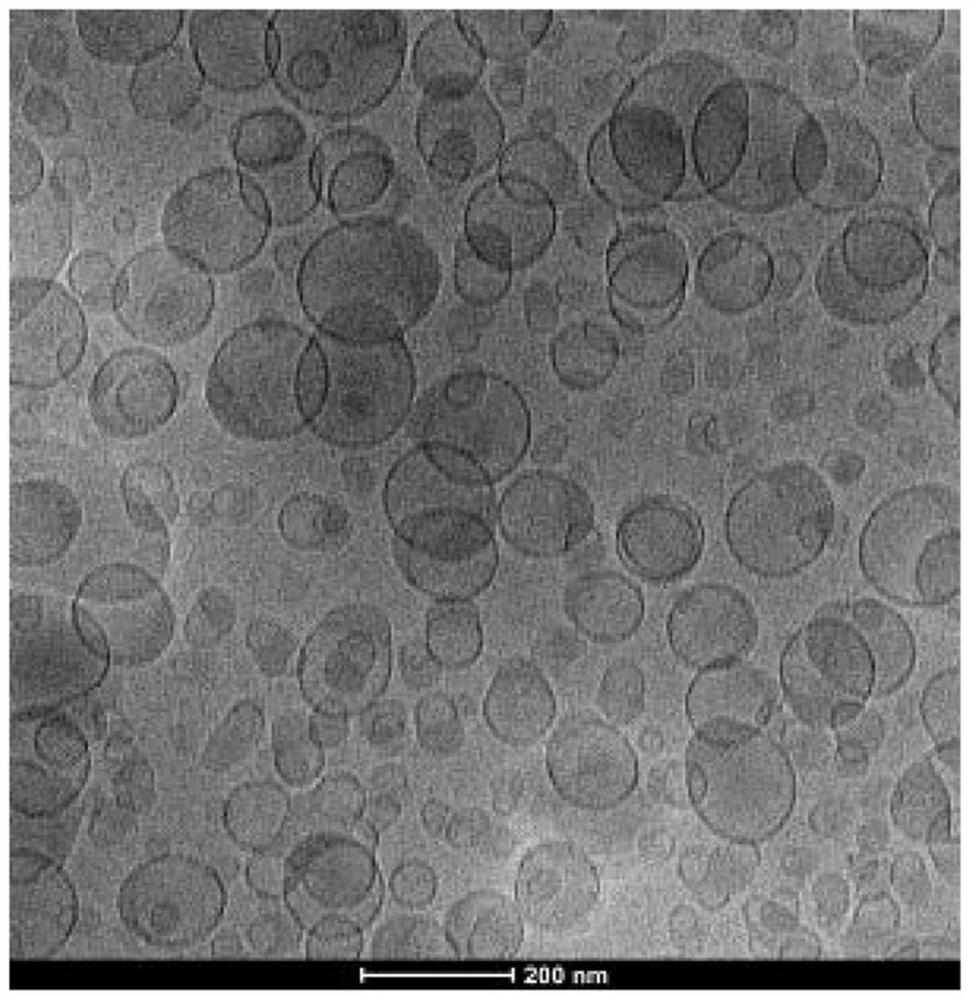
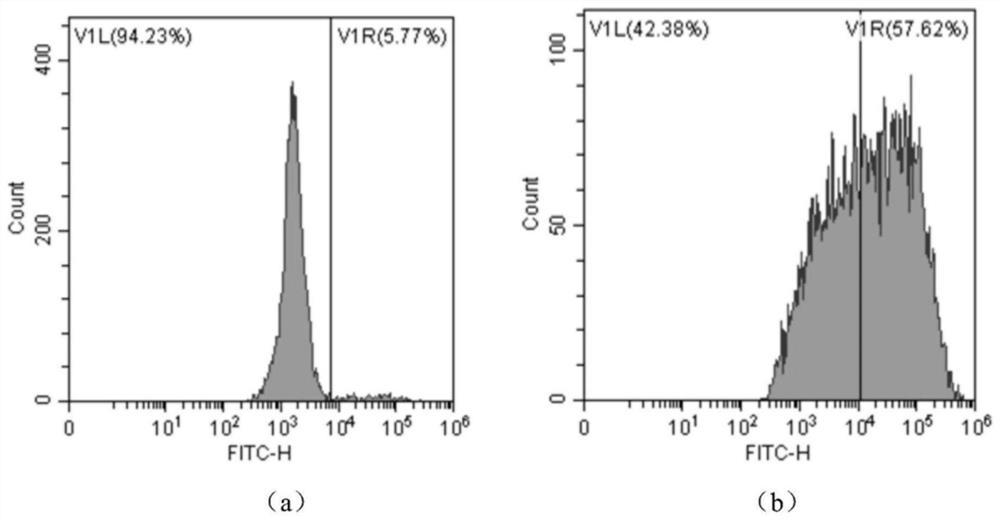
Image
Examples
Embodiment 1
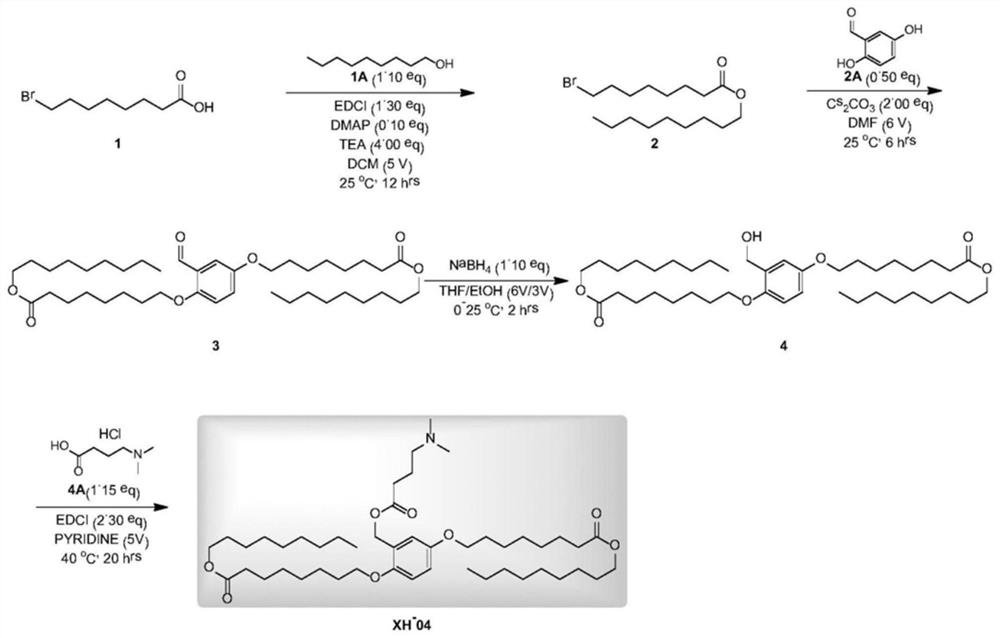
[0107] The synthesis of embodiment 1 compound one (code name: XH-04)
[0108] (Compound 1, code name: XH-04)
[0109] The preparation method of compound one is as figure 1 Shown; specifically include the following steps:
[0110] Step 1, compound 1 in DCM dichloromethane (100mL) (20.0g, 89.6mmol, 1.00 equiv) was added to the solution of compound 1A (14.2 g, 98.6 mmol, 1.10 equiv), DMAP4-dimethylaminopyridine (1.10 g, 8.96 mmol, 0.10 equiv), and TEA triethylamine (36.3 g, 358.6 mmol, 49.9 mL, 4.00 equiv). To the solution was added EDCI 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (22.3 g, 116.5 mmol, 1.30 equiv). The reaction was stirred at 25°C for 12 hours. TLC silica gel plate thin layer chromatography (petroleum ether:ethyl acetate=10:1, Rf=0.62) showed that the reaction was complete. The reaction solution was poured into H 2 O (100mL). The solution was extracted with dichloromethane (200 mL×2). The organic layer was washed with brine (100 mL). Th...
Embodiment 2
[0122] The synthesis of embodiment 2 compound two
[0123] Compound two:
[0124] Add triethylamine (40.8g) and dimethylaminopyridine (1.64g) and 1-ethyl-(3-di Methylaminopropyl) carbodiimide hydrochloride (25.7 g), stirred at room temperature for 12 h, then added hydrochloric acid (20 mL) solution to terminate the reaction. Extracted 3 times with ethyl acetate, and washed the filtrate with brine. Dry over anhydrous magnesium sulfate, filter and remove the solvent, and obtain 14 g of nonyl-8-bromooctanoic acid ethyl ester after purification by chromatography column.
[0125] Add triethylamine (68g) and dimethylaminopyridine (2.74g) and 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (94.5g), stirred at room temperature for 12h, then added hydrochloric acid solution to terminate the reaction, then extracted 3 times with ethyl acetate, And the filtrate was washed with brine. Dry over anhydrous magnesium sulfate, filter and remove the solvent, and obtain a colorl...
Embodiment 3
[0129] Embodiment 3: the synthesis of compound three
[0130] Compound three:
[0131] The synthesis of compound three refers to the synthetic method of compound two. The H NMR spectrum of compound three is as follows:
[0132] 1 H NMR: (400MHz, CDCl 3 )δ6.86(s,1H),6.79(s,2H),5.15(s,2H),4.86(t,J=6.2Hz,1H),4.06(t,J=6.6Hz,2H),3.91( td,J=6.4,9.0Hz,4H),3.09-2.91(m,2H),2.74(s,6H),2.52(t,J=6.6Hz,2H),2.32-2.27(m,4H),2.23 -2.10(m,2H),1.79-1.74(m,4H),1.65-1.58(m,6H),1.55-1.41(m,9H),1.41-1.34(m,9H),1.33-1.23(m, 34H), 0.93-0.83 (m, 9H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com