Safe and efficient degradable lipid nanoparticles as well as preparation method and application thereof
A technology selected from and compounds, applied in the field of degradable lipid nanoparticles and its preparation, can solve the problems of the effective delivery of nucleic acids with physical and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
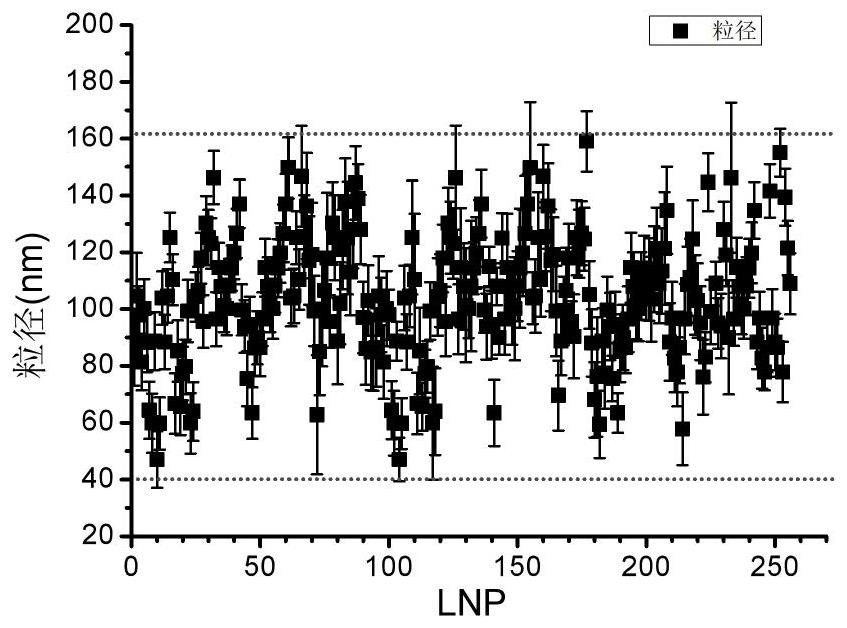
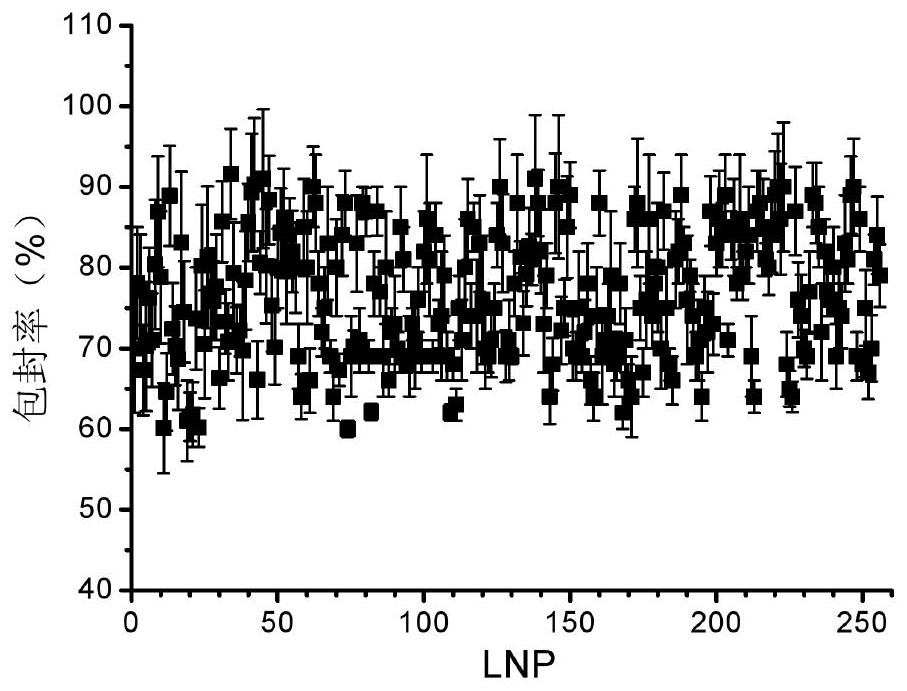
Image
Examples
preparation example Construction
[0068] The reaction formula of the preparation of degradable lipid nanoparticles of the present invention is as follows:
[0069]
[0070] It should be noted that the above reaction formula is just a Michael addition reaction between a typical double bond chemical (II) and an NH-containing structure (B-AH). When A or B additionally contains NH or B is H, the reaction occurs and follows the reaction mechanism here.
Embodiment 1
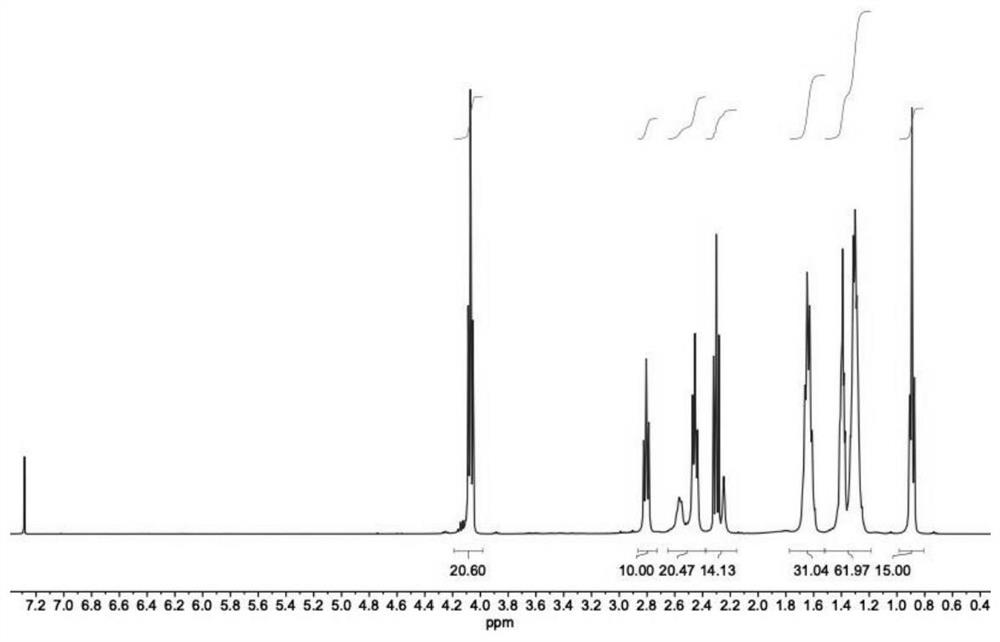
[0073] The synthesis of Lipidoid 53 includes the following steps:
[0074]
[0075] 6-Hydroxyhexyl acrylate (17.2 g, 0.1 mol), octanoic acid (17.3 g, 0.12 mol), EDC HCl (21.5 g, 0.12 mol), 4-dimethylaminopyridine (DMAP, 1.46 mol) were added to a round-bottomed flask. g, 0.012 mol) 300 mL of dichloromethane, and the reaction was stirred at room temperature for 12 hours. After the reaction, washed with dilute hydrochloric acid solution (1 wt %, 300 mL×2), water (300 mL×2) and saturated sodium bicarbonate solution (300 mL×2) successively. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (SiO 2 , n-hexane:dichloromethane=1:0 to 0:1) and purified to give compound 2 (20.8 g, 70%) as a yellow oil.
[0076] A (17.9 g, 0.06 mol) and diethylenetriamine (1.03 g, 0.01 mol) were sequentially added to a round-bottomed flask, and the reaction was stirred a...
Embodiment 2
[0078] The synthesis of Lipidoid 41 includes the following steps:
[0079]
[0080] 4-Hydroxybutylacrylamide (14.3g, 0.1mol), nonanoic acid (19g, 0.12mol), EDC HCl (21.5g, 0.12mol), 4-dimethylaminopyridine (DMAP, 1.46 g, 0.012 mol) 300 mL of dichloromethane, and the reaction was stirred at room temperature for 12 hours. After the reaction, washed with dilute hydrochloric acid solution (1 wt %, 300 mL×2), water (300 mL×2) and saturated sodium bicarbonate solution (300 mL×2) successively. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (SiO 2 , dichloromethane:methanol=1:0 to 5:1) was purified to give compound 2 (20.2 g, 71%) as a yellow oil. Then, A (13.7 g, 0.048 mol) and amino-tripolyethylene glycol (1.48 g, 0.01 mol) were sequentially added to the round-bottomed flask, and the reaction was stirred at 80°C for 48 hours. After the reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com