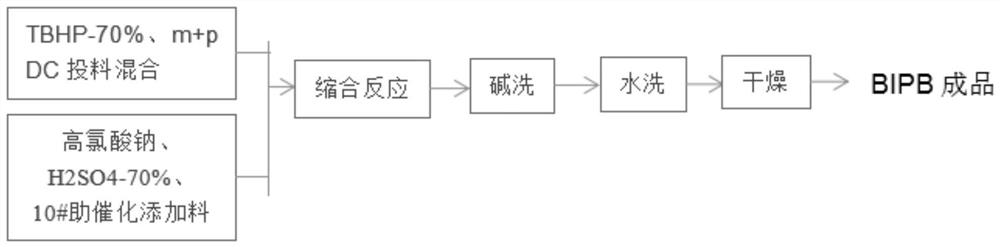
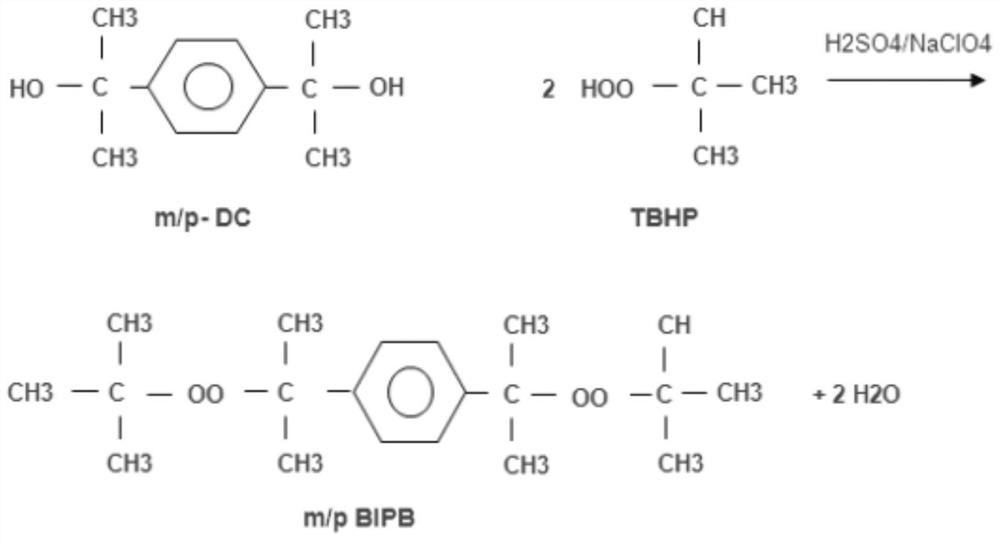
Condensation reaction method for synthesizing meta-position and para-position 2-(tert-butylperoxyisopropyl)benzene
A technology of isopropyl tert-butyl peroxide and tert-butyl hydroperoxide, which is applied in the field of chemical synthesis of organic peroxides, can solve problems such as low boiling point of solvent oil, process safety problems, raw material loss production, etc., and achieve reduction Usage amount, avoiding liquid caustic soda loss and production waste, and saving consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] In order to further understand the content, characteristics and effects of the present invention, the following examples are given, and detailed descriptions are given below with reference to the accompanying drawings.
[0025] The structure of the present invention will be described in detail below in conjunction with the accompanying drawings.
[0026] Such as Figure 1 to Figure 2 As shown, a condensation reaction method for synthesizing meta+para 2-(tert-butylperoxyisopropyl)benzene provided by the embodiment of the present invention, the synthesis reaction process is as follows: step S1, first according to the feeding ratio 2-cumyl alcohol m+pDC: tert-butyl hydroperoxide TBHP = 1: 2.3 ~ 2.8 (mol ratio), the raw material 70%-TBHP is metered into the condensation reactor, fully stirred, and the temperature is kept at 30°C ±5°C;
[0027] Step S2, in a fully stirred state, metering the raw material m+pDC into the condensation reactor, fully stirring, and keeping the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com