Method for synthesizing cytidine diphosphate
A technology of cytidine diphosphate and cytidine monophosphate, which is applied in the field of synthesizing cytidine diphosphate, can solve the problems of impurity products, difficult separation and purification, etc., and achieves the effects of stable yield, simple process operation and reduction of waste generation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
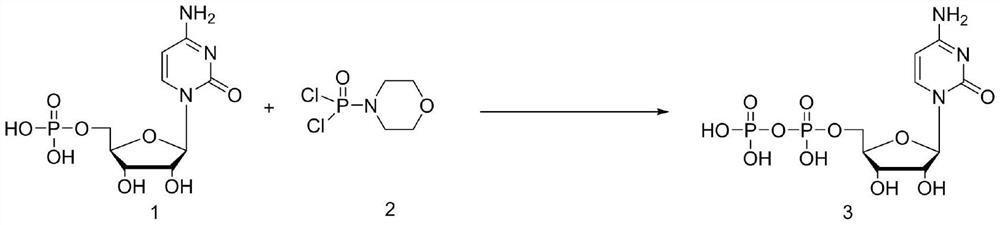
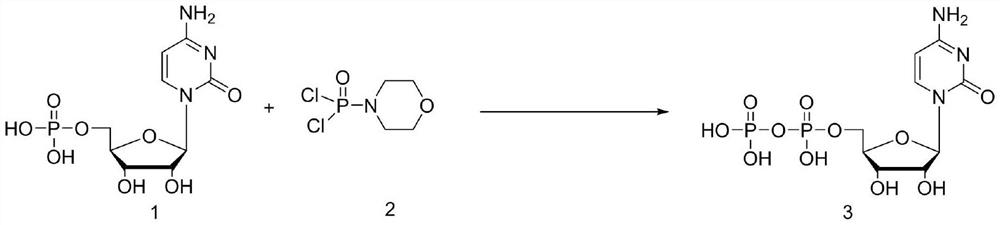
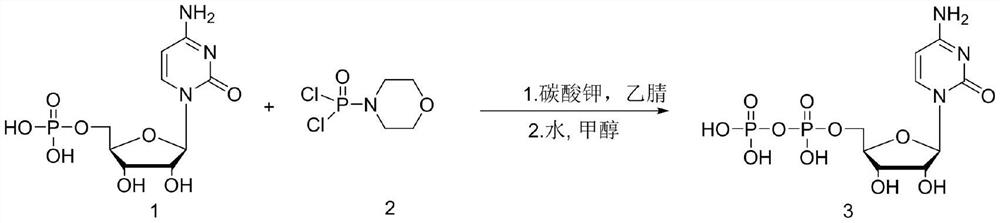
[0027] In a three-neck flask equipped with a stirring device, add 200 mL of acetonitrile, 20 g of cytidylic acid (0.062 mol) and potassium carbonate (21.4 g, 0.155 mol) in sequence, stir evenly, cool down to 0 ° C, and slowly add dichlorophosphoryl morpholine dropwise (13.19g, 0.062mol), continued to stir for 2 hours after the dropwise addition, followed the reaction by HPLC until the residual cytidylic acid raw material was less than 2%, lowered to 0°C, and removed the salt generated during the reaction by suction filtration. Concentrate the filtrate to oily state, add 40mL of water to stir and hydrolyze, then add 100mL of methanol dropwise, during the process, solids are precipitated, after the dropwise addition is completed, stir at 20°C for 3h, and when the white solid is completely precipitated, filter and dry with suction to obtain 23.8g (0.059mol) of crude product Cytidine diphosphate, HPLC purity 92.1%, yield 95.2%.
[0028] Put all the above crude cytidine...
Embodiment 2
[0030]
[0031] In a three-neck flask equipped with a stirring device, add 200mL of dioxane, 20g of cytidylic acid (0.062mol) and potassium carbonate (21.4g, 0.155mol) in sequence, stir evenly, cool down to 0°C, and slowly add dichlorophosphoryl Morpholine (13.19g, 0.062mol) was added dropwise and continued to stir for 2 hours. HPLC followed the reaction until the residual cytidylic acid raw material was less than 2%, lowered to 0°C, and filtered to remove the salt formed during the reaction. Concentrate the filtrate to an oily state, add 40mL of water to stir and hydrolyze, then add 100mL of methanol dropwise, during the process, solids are precipitated, after the dropwise addition is completed, stir at 20°C for 3h, and when the white solid is completely precipitated, filter and dry with suction to obtain 21.2g (0.052mol) of crude product Cytidine diphosphate, HPLC purity 91.1%, yield 84%.
[0032] Put all the above crude cytidine diphosphate into a three-necked flask, the...
Embodiment 3
[0034]
[0035] In a three-necked flask equipped with a stirring device, add 200mL of acetonitrile, 20g of cytidylic acid (0.062mol) and triethylamine (15.7g, 0.155mol) in sequence, stir evenly, cool down to 0°C, and slowly add dichlorophosphoryl Phenyl (13.19g, 0.062mol), continue to stir for 2 hours after adding dropwise, follow the reaction by HPLC until the residual cytidylic acid raw material is less than 2%, drop to 0°C, and remove the salt formed during the reaction by suction filtration. Concentrate the filtrate to oily state, add 40mL of water to stir and hydrolyze, then add 100mL of methanol dropwise, during the process, solids are precipitated, after the dropwise addition is completed, stir at 20°C for 3h, and when the white solid is completely precipitated, filter and dry with suction to obtain 22.8g (0.057mol) of crude product Cytidine diphosphate, HPLC purity 92.3%, yield 91.9%.
[0036] Put all the above crude cytidine diphosphate into a three-necked flask, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com