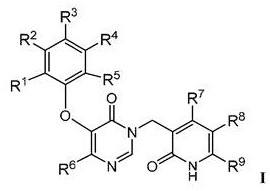
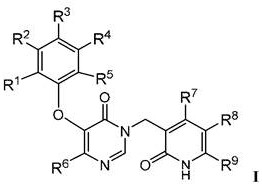
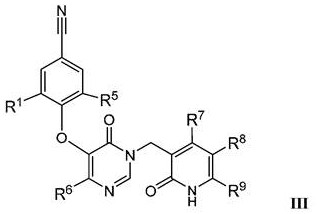
Pyridinone derivatives as selective cytotoxic agents against HIV infected cells
A technology of dihydropyridine and compound, applied in the direction of antiviral agents, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of virus resistance, rapid rebound of viremia, and incurable solutions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0426]
[0427] 5-Chloro-2-fluoro-3-((1-((6-(methoxymethyl)-2-oxo-1,2-dihydropyridin-3-yl)methyl)-6-oxo Substituent-4-(1,1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile
[0428] Step 1: 5-Chloro-2-fluoro-3-((1-((2-methoxy-6-(methoxymethyl)pyridin-3-yl)methyl)-6-oxo Substituent-4-(1,1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile
[0429] To 3-(chloromethyl)-2-methoxy-6-(methoxymethyl)pyridine, C15 (30 mg, 0.149 mmol), 5-chloro-2-fluoro-3-((6-oxo Dioxo-4-(1,1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile AB04 (35 mg, 0.086 mmol) in DMF (1 mL) K was added to the stirred solution of 2 CO 3 (23.81 mg, 0.172 mmol) and lithium bromide (11.22 mg, 0.129 mmol). The resulting mixture was stirred at 15 °C for 1 h. TLC showed that the reaction was complete and the mixture was washed with H 2 Dilute with O (10 mL) and extract with EtOAc (3×10 mL). The combined organic layers were washed with brine (10 mL), washed wi...
Embodiment 14
[0438]
[0439] 5-(difluoromethyl)-3-((1-((5-fluoro-2-oxo-1,2-dihydropyridin-3-yl)methyl)-6-oxo-4-( 1, 1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)-2-methylbenzonitrile
[0440] Step 1: 5-(Difluoromethyl)-3-((1-((5-fluoro-2-oxo-1,2-dihydropyridin-3-yl)methyl)-6-oxo Substitute-4-(1,1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)-2-methylbenzonitrile
[0441] To 5-(difluoromethyl)-2-methyl-3-((6-oxo-4-(1,1,2,2-tetrafluoroethyl)-1,6-dihydropyrimidine at room temperature -5-yl)oxy)benzonitrile, AB01 (30 mg, 0.080 mmol) and 3-(chloromethyl)-5-fluoro-2-methoxypyridine, C20 (15.36 mg, 0.087 mmol) in NMP ( 795 µL) was added potassium carbonate (21.98 mg, 0.159 mmol). The resulting mixture was stirred at room temperature overnight, then diluted with water (3 mL), and extracted with EtOAc (3 mL). The organic layer was concentrated under reduced pressure to give the title compound which was used in the next step without further purification. MS: 517.1 (M+1...
Embodiment 21
[0448]
[0449] 5-chloro-2-fluoro-3-((1-((6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-6-oxo-4-( Complete Fluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile
[0450] Step 1: 5-Chloro-2-fluoro-3-((1-((2-methoxy-6-methylpyridin-3-yl)methyl)-6-oxo-4-(perfluoro Ethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile
[0451] To 5-chloro-2-fluoro-3-((6-oxo-4-(perfluoroethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile, AB06 (50 mg, 0.130 mmol) and (2-methoxy-6-methylpyridin-3-yl)methanol, C17 (20.0 mg, 0.13 mmol) in DCM (1303 µL) was added dropwise to an ice-cold solution of DIAD (25.3 µL, 0.130 mmol). The resulting mixture was warmed to room temperature, then washed with saturated NaHCO 3 The aqueous solution was diluted and extracted with DCM. The organic layer was washed with anhydrous MgSO 4 Dry, filter and concentrate under reduced pressure. The residue was purified by column chromatography on silica (0-100% EtOAc / hexanes) to give the title compound. MS: 519....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com