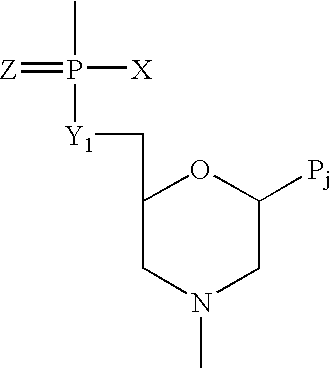
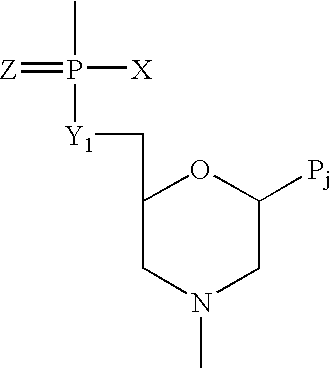
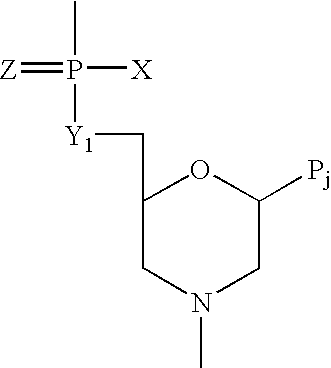
Method and antisense composition for selective inhibition of HIV infection in hematopoietic cells
a technology of hematopoietic cells and antiviral conjugates, applied in the field of new drugs, can solve the problems of no curative anti-retroviral drugs against aids, emergence of viral resistance, etc., and achieve the effect of a greater intracellular uptak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of rTAT-Antisense Conjugates
[0146] A. Production of PMO and Peptide Conjugated PMOs.
[0147] The PMOs were synthesized at AVI BioPharma (Corvallis, Oreg.) as previously described (Summerton and Weller, 1997). Purity of full length oligomers was >95% as determined by reverse-phase high-pressure liquid chromatography (HPLC) and MALDI TOF mass spectroscopy. Peptide conjugated forms of the PMO where produced by attaching the carboxy terminal cysteine of the peptide to the 5′ end of the PMO through a cross-linker N-[□-maleimidobutyryloxy] succinimide ester (GMBS) (Moulton and Moulton, 2003), as detailed below in section C. The peptides used in this study designated as P002 (RRRQRRKKRC, SEQ ID NO:1) (Moulton and Moulton, 2003) and P003 (RRRRRRRRRFFC, SEQ ID NO:2). The lyophilized PMO or peptide-conjugated PMO were dissolved in sterile H2O prior to use in cell cultures or dilution in PBS prior to injection in to mice.
[0148] B. 3′-Fluoresceination of a PMO (Phosphorodiamidate-L...
example 2
Uptake of rTAT-Antisense Conjugates Selectively into Activated T Cells
[0158] The DO11.10 transgenic mouse system (Murphy, Heimberger et al. 1990) was used as a source of splenocytes and T cells. This transgenic mouse contains the gene for the T cell receptor (TCR) from the T cell hybridoma, DO11.10, that recognizes chicken ovalbumin (OVA). Virtually all thymocytes and peripheral T cells in these mice express the OVA-TCR which is detected by the KJ26 monoclonal antibody.
[0159] A. Uptake in Naïve and Activated Murine Lymphocytes
[0160] Freshly isolated splenocytes from B6 mice were plated (1.5 million / well) into 96 well V-bottom plates and incubated with PMO-fl, P002-PMO-fl or P003-PMO-fl [10 μM, 10 μM and 5 μM in culture media, respectively]. Lymphocyte activating substances derived from bacterial (LPS), murine cytokine (Gamma IFN), mitogenic plant lectin (PHA), chemical activator (PMA+ION) or culture media control (naïve cell treatment) were added to individual cultures as follows...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com