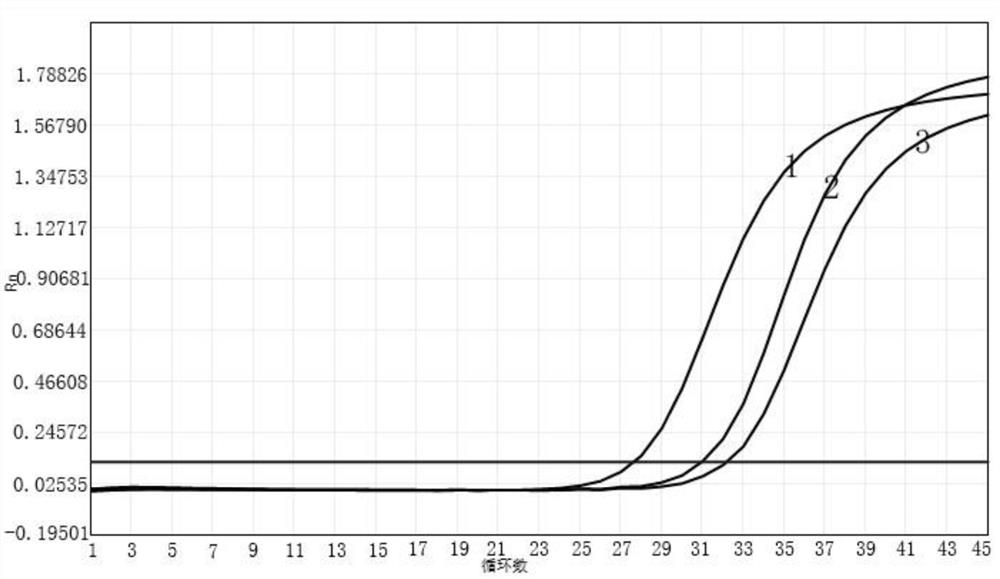
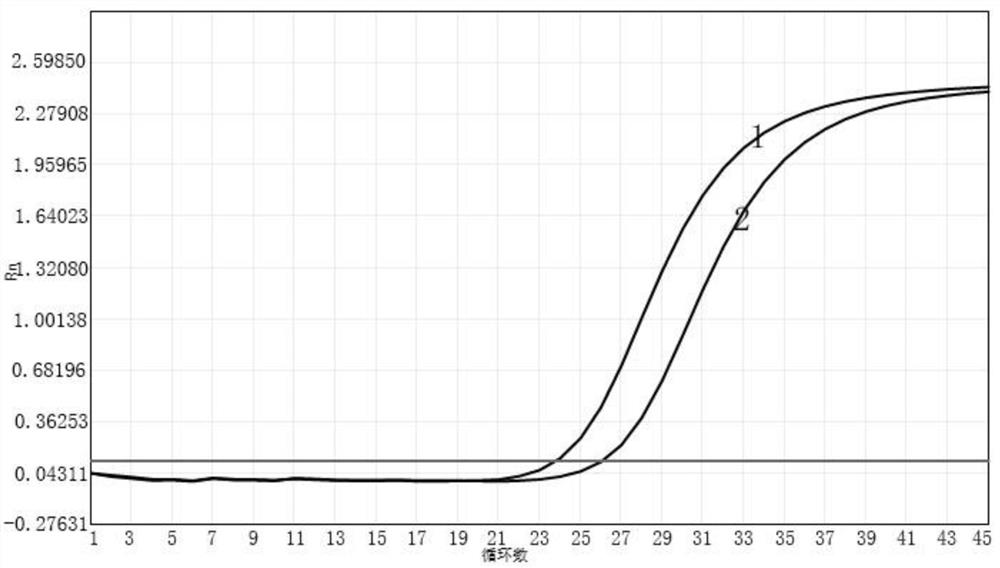
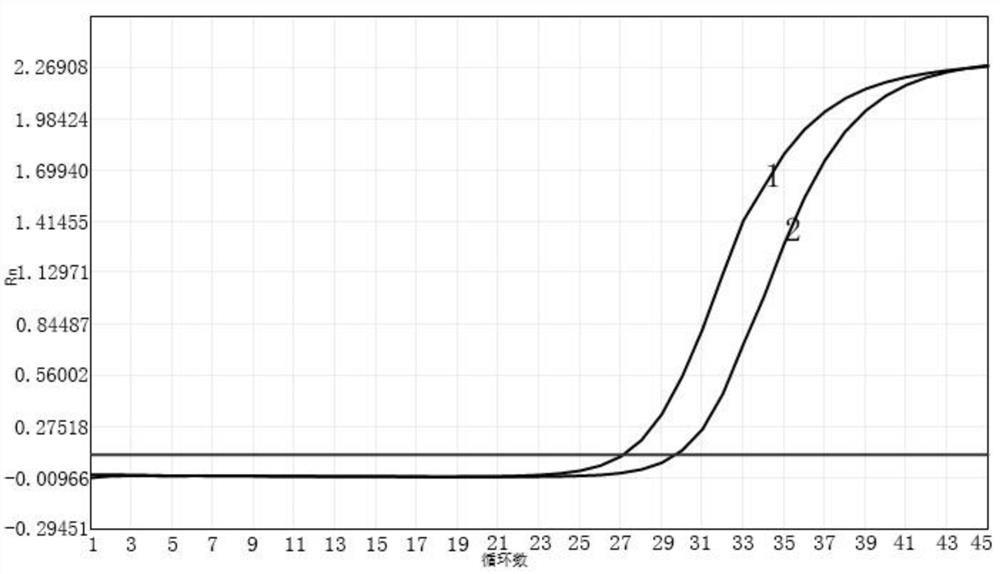
Primer probe composition, kit containing primer probe composition and detection method of primer probe composition
A primer-probe and kit technology, which is used in the determination/inspection of microorganisms, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problems of low detection efficiency, poor specificity and low sensitivity of the kit, and achieve accurate detection. High sensitivity and sensitivity, accurate results and high detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The primer-probe composition for detecting influenza A virus, influenza B virus and respiratory syncytial virus, and the preparation of the kit comprising it are as follows:
[0047] The primer and probe sequences in the kit are shown in Table 1 below:
[0048] Table 1 Primer, probe sequence set
[0049]
[0050] In this embodiment, the 5' end of the probe shown in SEQ ID NO:3 is marked with a VIC fluorescent group, and the 3' end is marked with a BHQ1 quenching group, so as to ensure that influenza A viruses can be distinguished during the reaction and improve detection efficiency.
[0051] In this embodiment, the 5' end of the probe shown in SEQ ID NO: 6 is marked with a ROX fluorescent group, and the 3' end is marked with a BHQ2 quenching group to ensure that influenza B viruses can be distinguished during the reaction and improve detection efficiency.
[0052]In this embodiment, the 5' end of the probe shown in SEQ ID NO: 9 is marked with a FAM fluorescent gro...
Embodiment 2
[0057] Methods for detecting influenza A virus, influenza B virus, and respiratory syncytial virus for non-diagnostic purposes
[0058] The detection method adopted in the present invention is q-PCR, and the PCR reaction solution, DNA polymerase, reverse transcriptase and sample nucleic acid are prepared into a nucleic acid amplification reaction mixture according to the proportion of the following reaction system for PCR reaction. The proportioning ratio of the reaction system is shown in Table 2:
[0059] Table 2 Configuration of nucleic acid amplification reaction mixture
[0060]
[0061] After the nucleic acid amplification reaction mixture is configured, the PCR reaction is carried out according to the procedure shown in Table 3:
[0062] Table 3 Fluorescent quantitative PCR reaction conditions
[0063]
[0064] Selection of fluorescence detection channels: select VIC channel to detect influenza A virus nucleic acid; select ROX channel to detect influenza B virus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com