Compounds and compositions for eye treatments
A compound and selected technology, applied in the direction of drug combination, compound of group 5/15 elements of the periodic table, treatment, etc., can solve the problem of weakening and reversing the expected effect of reshaping the cornea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
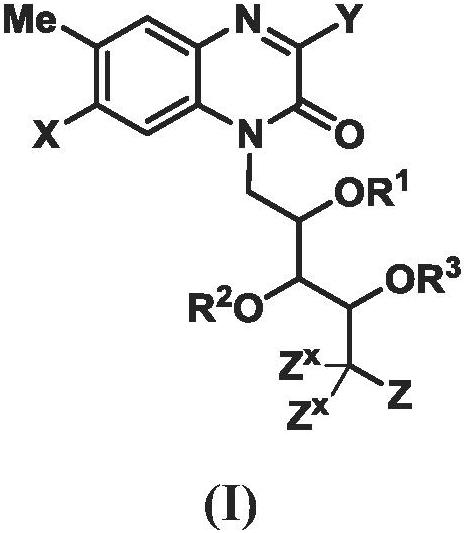
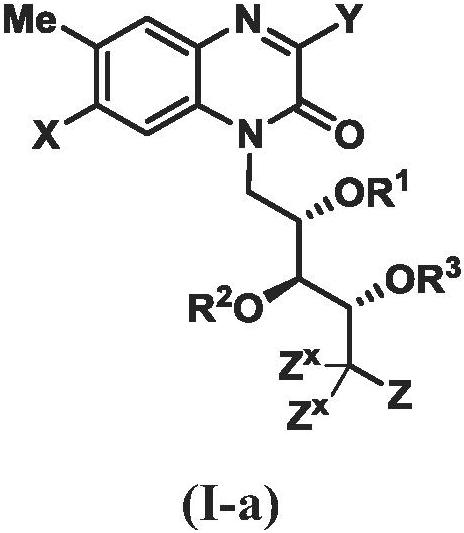
[0802] In certain embodiments of [1]-[7], Z is as defined in claims 6-15.
[0803] In certain embodiments of [1]-[7], Z is as defined in claim 6.
[0804] In certain embodiments of [1]-[7], Z is as defined in claim 7 (i.e., Z is NR 4Z R 5Z ).
[0805] In some of these embodiments, R 4Z and R 5Z as defined in claim 8. In some of these embodiments, R 4Z and R 5Z as defined in claim 9. In some of these embodiments, R 4Z and R 5Z as defined in claim 10. In some of these embodiments, R 4Z and R 5Z as defined in claim 11. In some of the foregoing embodiments, when R 4Z and R 5Z each independently selected from H and when R a as defined in claim 12. In some embodiments of [1]-[7], R 4Z and R 5Z each independently selected from H and
[0806] In certain embodiments of [1]-[7], Z is NR 4Z R 5Z ; and R 4Z and R 5Z as defined in claim 14.
[0807] In certain embodiments of [1]-[7], Z is NR 4Z R 5Z ; and R 4Z and R 5Z as defined in claim 15.
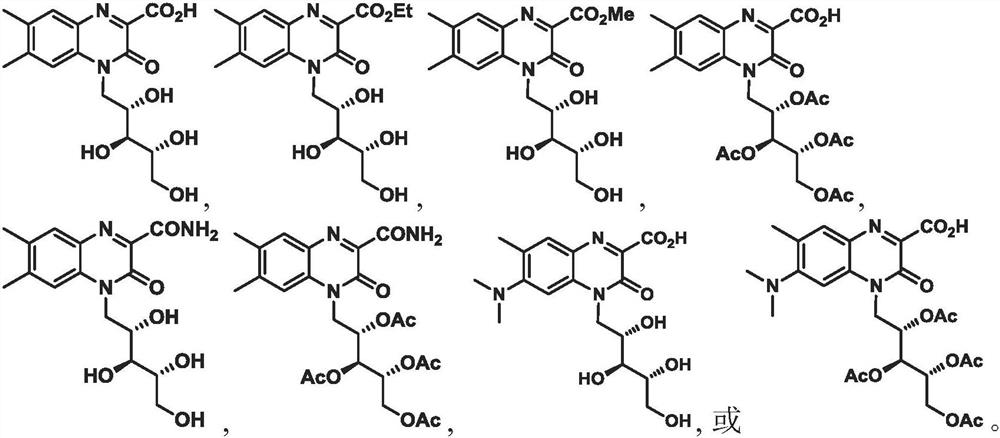
[0808] In c...
Embodiment 1
[1136] To a suspension of compound (2) (1.5 g, 4.3 mmol) in 100 mL of MeOH was added neat SOCl dropwise at ambient temperature 2 (0.51 g, 4.3 mmol). The reaction mixture was stirred overnight at reflux and evaporated to dryness. The residue was passed through a pad of silica gel eluting with 20% MeOH in chloroform to afford 1.0 g of compound (3) (Example 1).
[1137] 1 H-NMR (DMSO-d 6 , δ, ppm): 2.30(s, 3H), 2.38(s, 3H), 3.41-3.49(m, 1H), 3.55-3.65(m, 3H), 4.07-4.14(m, 1H), 4.18(br .d, 1H, J = 14.0Hz), 4.47 (dd, 1H, J 1 =J 2 =5.8Hz), 4.59(dd,1H,J 1 = 3.6Hz,J 2 = 14.0 Hz), 4.72 (d, 1H, J = 5.8 Hz), 4.82 (d, 1H, J = 2.7 Hz), 4.97 (d, 1H, J = 4.3 Hz), 7.61 (br.s, 2H).
[1138] 13 C-NMR (DMSO-d 6 , δ, ppm): 18.63, 20.33, 44.50, 52.61, 63.51, 68.60, 72.76, 73.83, 116.26, 129.66, 129.96, 132.34, 132.68, 142.20, 147.65, 152.41, 164.54.
[1139] LCMS, m / z: 367.0 (M+H) + , 389.5 (M+Na) + .
[1140] Compound 4 (Example 5)
[1141] Compound (2) (2.5 g, 7.1 mmol) was susp...
Embodiment 2
[1161] Compound (9) (3.6 g, 7.7 mmol) was dissolved in MeCN (50 mL). The solution was purged with nitrogen, and Pd(PPh 3 ) 4 (0.89 g, 0.77 mmol), followed by addition of pyrrolidine (0.66 g, 9.2 mmol). After 16 hours at room temperature, MeCN was evaporated, EtOAc was added and the particles formed were filtered off and washed with EtOAc. The particles were dissolved in water and the pH was adjusted to 2 by adding 10% HCl. The particles formed were filtered off, washed with water and dried, yielding 1.56 g of compound (10) (Example 2).
[1162] 1 H-NMR (DMSO-d 6 , δ, ppm): 1.10 (dd, 6H, J 1 = 1.7Hz,J 2 =7.1Hz), 2.32(s, 3H), 2.40(s, 3H), 2.52-2.59(m, 1H), 3.60-3.62(m, 1H), 3.81-3.88(m, 1H), 4.02(dd, 6H,J 1 =7.0Hz,J 2 =10.9Hz), 4.10-4.12(m, 1H), 4.20-4.30(m, 2H), 4.58(dd, 6H, J 1 =10.2Hz,J 2 =13.7Hz), 4.82-4.89 (m, 1H), 5.12-5.23 (m, 2H), 7.60 (s, 1H), 7.65 (s, 1H), 13.98 (br.s, 1H).
[1163] 13 C-NMR (DMSO-d 6 , δ, ppm): 18.67, 18.91 (2×), 20.38, 33.31, 44.84, 66...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com