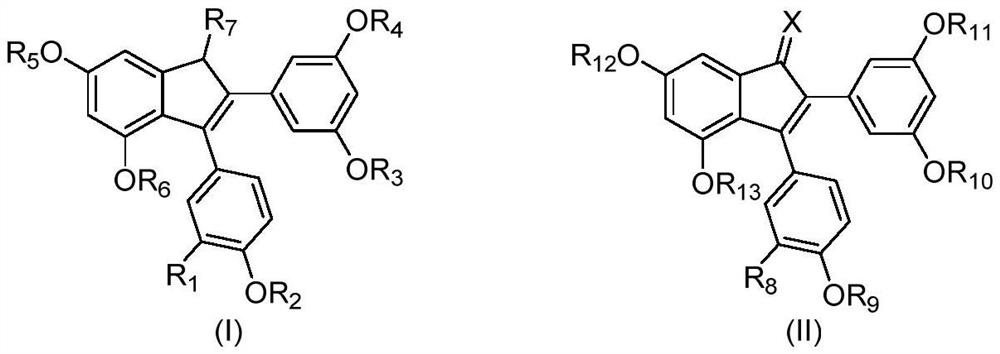
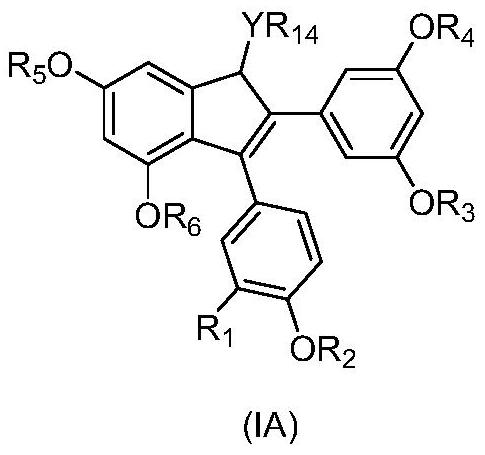
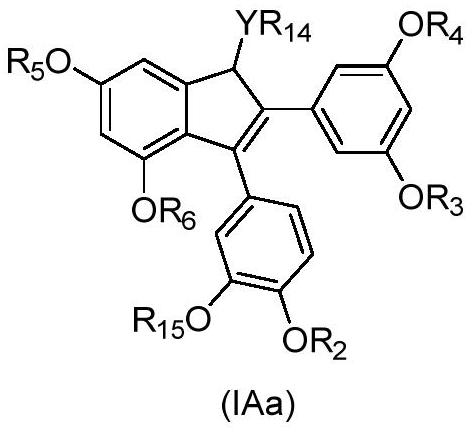
2,3-diarylindene derivatives, and preparation method, pharmaceutical compositions and application thereof
A technology of derivatives and aryl indene, applied in the direction of drug combination, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of lack of samples, low content, few synthesis and activity research reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0113] In order to further clarify the present invention, a series of examples are given below, these examples are completely illustrative, they are only used to describe the present invention in detail, and should not be construed as limiting the present invention.
[0114] The synthetic route of intermediate compound 1a in the embodiment:
[0115]
Embodiment 1
[0117] 2-(3,5-dimethoxymethoxyphenyl)-3-(3-methoxy-4-methoxymethoxyphenyl)-4,6-dimethoxymethoxy-1H- Inden-1-one (1)
[0118] The synthetic route of compound 1:
[0119]
[0120] Compound 1a (5g, 9.0 12.0mmol) was dissolved in 10ml of toluene, followed by adding compound 1g (8.8g, 24.0mmol), palladium acetate (112.5mg, 0.4 0.5mmol), xphos (333.2mg, 0.7mmol) and tert-butanol Sodium (1.73g, 18mmol). React in a sealed bottle at 120° C. for 3 h, TLC detection shows that the reaction is complete, and the reaction is stopped. The reaction solution was cooled to room temperature, diluted with 50ml of water, filtered with diatomaceous earth, extracted with ethyl acetate, the organic phases were combined, washed three times with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 200-300 mesh silica gel column chromatography, eluting with petroleum ether: dichloromethane: acetone (...
Embodiment 2
[0123] 2-(3,5-dihydroxyphenyl)-3-(3-methoxy-4-hydroxyphenyl)-4,6-dihydroxy-1H-inden-1-one (2)
[0124] Synthetic routes of compounds 2 and 3:
[0125]
[0126] Compound 1 (100 mg, 0.163 mmol) was dissolved in 10 ml of methanol, 25 N concentrated hydrochloric acid was added, and reacted at room temperature for 10 h. TLC detection showed that the reaction was complete. Adjust the pH of the reaction solution to neutral with saturated sodium bicarbonate solution, add 20ml of water to dilute, extract with ethyl acetate, combine the organic phases, wash with saturated sodium chloride solution, dry over anhydrous magnesium sulfate, filter under reduced pressure, and decompress the filtrate concentrate. The resulting solid was chromatographed on a 200-300 mesh silica gel column and eluted with dichloromethane:methanol (10:1) to give colorless oily liquids 2 (19 mg, 30%) and 3 (9 mg, 12%).
[0127] Compound 2: black solid: 1 H NMR (400MHz, Acetone-d 6 )δ: 8.90-7.87 (5H), 7.06 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com