Application of Apelin in preparation of medicine for treating silicosis
A drug and pharmaceutical technology, applied in the field of pharmaceutically active peptides, achieves the effects of good safety, alleviating fibrosis symptoms, and reducing expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
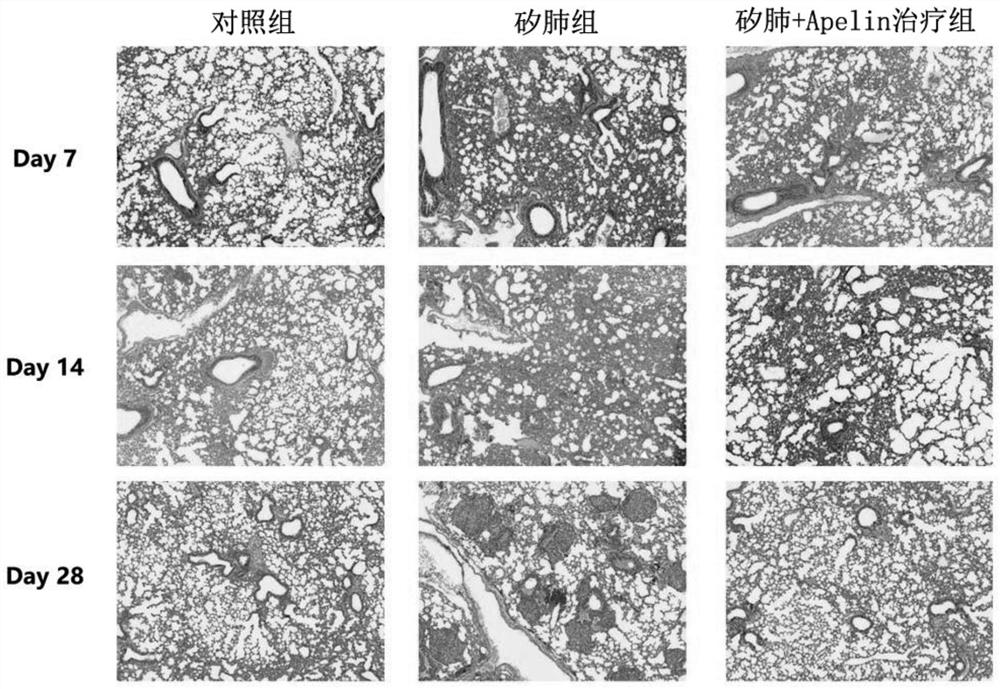
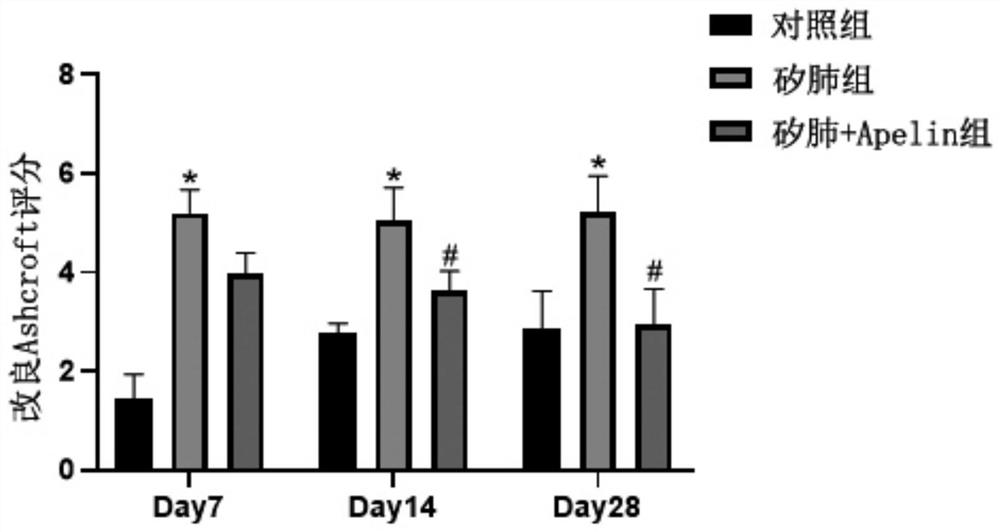
[0026] Embodiment 1: Therapeutic effect of Apelin on mouse silicosis
[0027] SPF grade C57BL / 6 male mice (6-8 weeks old, body weight 18-25g) were selected, randomly divided into 3 groups (15 mice in each group), and the control group, silicosis group and silicosis + Apelin treatment were respectively set up by the following methods Group.
[0028] Control group: Inject 20 μL of normal saline through the mouse trachea;
[0029] Silicosis group: Inject 20 μL of 250 g / L silica suspension through the trachea of mice by tracheal exposure method;
[0030] Silicosis + Apelin treatment group: Inject 20 μL of 250 g / L silica suspension through the trachea of mice by the tracheal exposure method. From the 3rd day after modeling, mice in this treatment group were intraperitoneally injected with 500 μg / kg of Apelin-13 trifluoroacetate (purchased from Germany sigma company).
[0031] After the above-mentioned groups were set up, conventional animal feeding was carried out; 5 mice in...
Embodiment 2
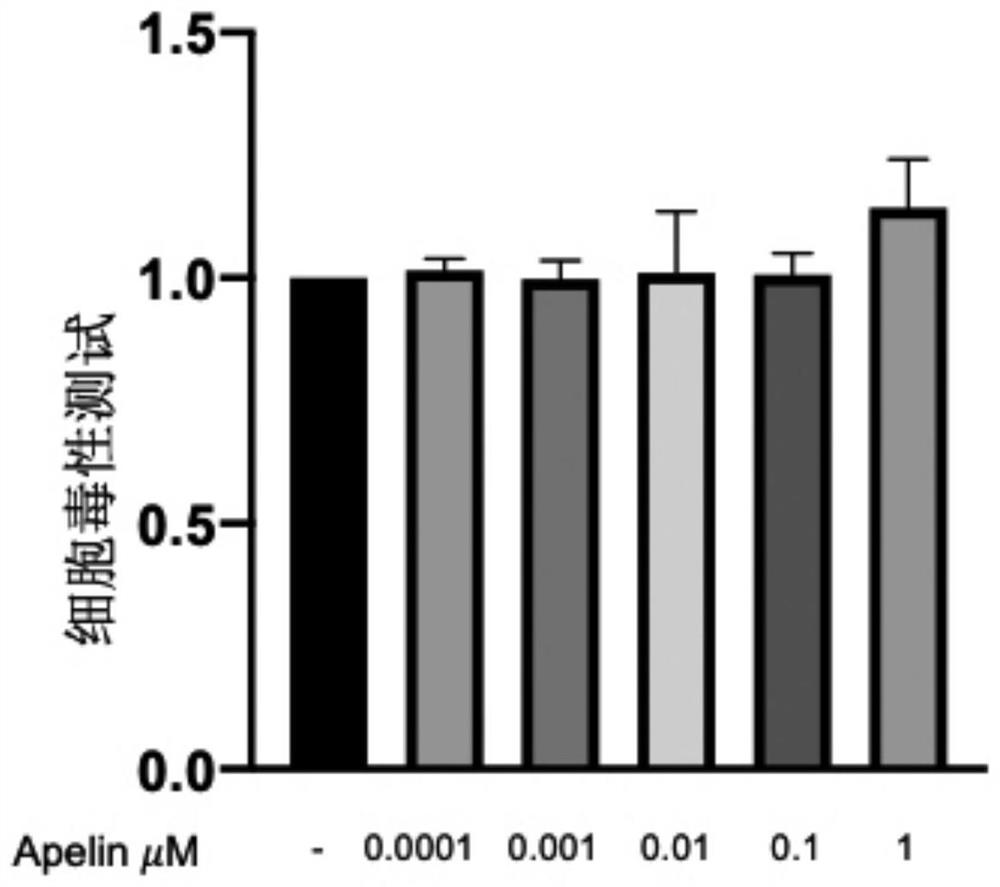
[0037] Embodiment 2: the influence of different concentrations of Apelin on A549 cytotoxicity
[0038] Control group: A549 cells (human lung cancer cells) were cultured in serum-free GIBCO DMEM high-glucose medium (purchased from Thermo Scientific, USA);
[0039]Apelin administration group: A549 cells (human lung cancer cells) were cultured in serum-free GIBCO DMEM high-glucose medium containing Apelin (Apelin-13 trifluoroacetate) at concentrations of 1, 0.1, 0.01, 0.001, and 0.0001 μM middle.
[0040] After the control group and the Apelin-administered group were placed in a constant temperature incubator for 48 hours, the toxic effects of Apelin at various concentrations on A549 were detected using the CCK-8 kit. Test results such as image 3 Shown: Compared with the control group, Apelin at 0.0001, 0.001, 0.01, and 0.1 μM has no obvious toxic effect on A549 cells.
Embodiment 3
[0041] Example 3: Effect of Apelin on expression level of epithelial-mesenchymal transition (EMT) marker protein regulated by silicon dioxide Control group: A549 cells (human lung cancer cells) were cultured in serum-free medium;
[0042] Silica exposure group: A549 cells (human lung cancer cells) were cultured in serum-free medium containing silica at a concentration of 100 μg / mL;
[0043] Apelin+silica exposure group: A549 cells (human lung cancer cells) were cultured in serum-free GIBCODMEM containing 100 μg / mL silica and 0.1, 1 μM Apelin (Apelin-13 trifluoroacetate) in high glucose medium.
[0044] The above groups were placed in a constant temperature incubator for 48 hours, and the changes in the protein levels of epithelial-mesenchymal transition (EMT) markers were detected by Western blotting. The detection results were as follows: Figure 4 shown.
[0045] Figure 4 The results showed that: compared with the control group, the protein expression of the epithelial m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com